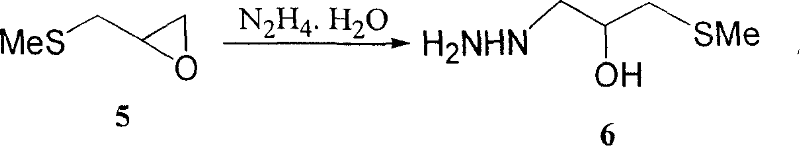Production method of nifuratel
A technology of nifuratel and compound is applied in the production field of antibiotic drug nifuratel, can solve problems such as reducing production cost, environmental pollution and the like, and achieves the effects of reducing production cost, reducing cost and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
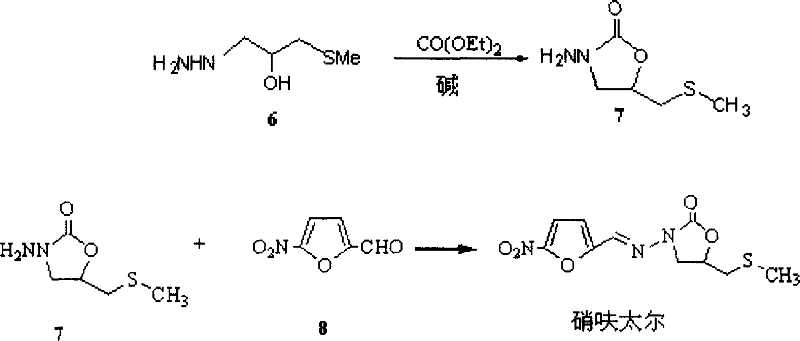
[0054] Preparation of Example 1 Intermediate 4
[0055] In a 2000mL reaction flask equipped with a high-efficiency reflux condenser and a dropping funnel, add 250g (3.125mol) of thiourea and 110mL of water, stir to partially dissolve the thiourea, stir for 10min under ice water cooling, remove the ice water, and add dropwise Dimethyl sulfate 15mL, the reaction spontaneously started under stirring, and exothermic, very violent at the beginning, after the reaction was stable, then slowly add the remaining dimethyl sulfate 155mL in the reaction bottle (about 1.5h), always Maintain a slightly boiling state, after the addition, continue to reflux for 1 hour, let it stand overnight, add 260 mL of 95% ethanol, stir for a while, cool, filter, wash twice with 95% ethanol, and dry to obtain 4364 g of product intermediates, with a yield of 84%. mp. 236°C (dec).
preparation Embodiment 2
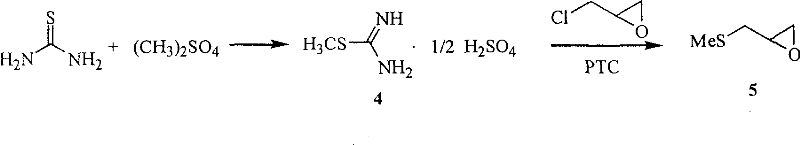
[0056] Preparation of Example 2 Intermediate 5
[0057] In the 2000mL reaction flask, add 4139g (1.0mol), epichlorohydrin 78.5mL (92.6g, 1.0mol), benzene 500mL, 30% sodium hydroxide 500mL and catalyst tetra-n-butylammonium bromide 6.4g, under efficient stirring React at room temperature for 6 hours, separate the organic layer, extract the aqueous phase with benzene (3×200mL), combine the organic phases, wash twice with water, dry over anhydrous magnesium sulfate, evaporate benzene at normal pressure (applicable), and distill the remaining liquid under reduced pressure , to obtain 561g of a colorless and transparent liquid product intermediate, b.p.54-56°C / 20mmHg (Lit.48-52°C / 20mmHg), yield 58.7%. IR (KBr, cm -1 ), 3045, 2985, 2914, 1424, 1260; MS: 105 (M+1).
preparation Embodiment 3
[0058] Preparation Example 3 Preparation of Intermediate 6
[0059] Add 200mL of hydrazine hydrate into the reaction flask, heat to 90°C, add 588.5g (0.85mol) dropwise with stirring, and control the internal temperature at about 90°C, complete the addition in about 0.5h, and continue the reaction at 90°C for 1h. Excess hydrazine and water were removed by rotary evaporation to obtain a viscous colorless liquid. It can be directly used in the next reaction without purification, and the yield is quantitative.
[0060] If the resulting crude product is purified by vacuum distillation (a part of the product is carbonized and decomposed due to high temperature during the vacuum distillation process), a colorless, transparent and viscous pure product intermediate 6 is obtained, with a yield of 50-60%, b.p.140-146 ℃ / 2-3mmHg, it can be solidified in the refrigerator for a few days.
[0061] Preparation of Example 4 Intermediate 7
[0062] method 1:
[0063] Add 693.5g (~0.688mol), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com