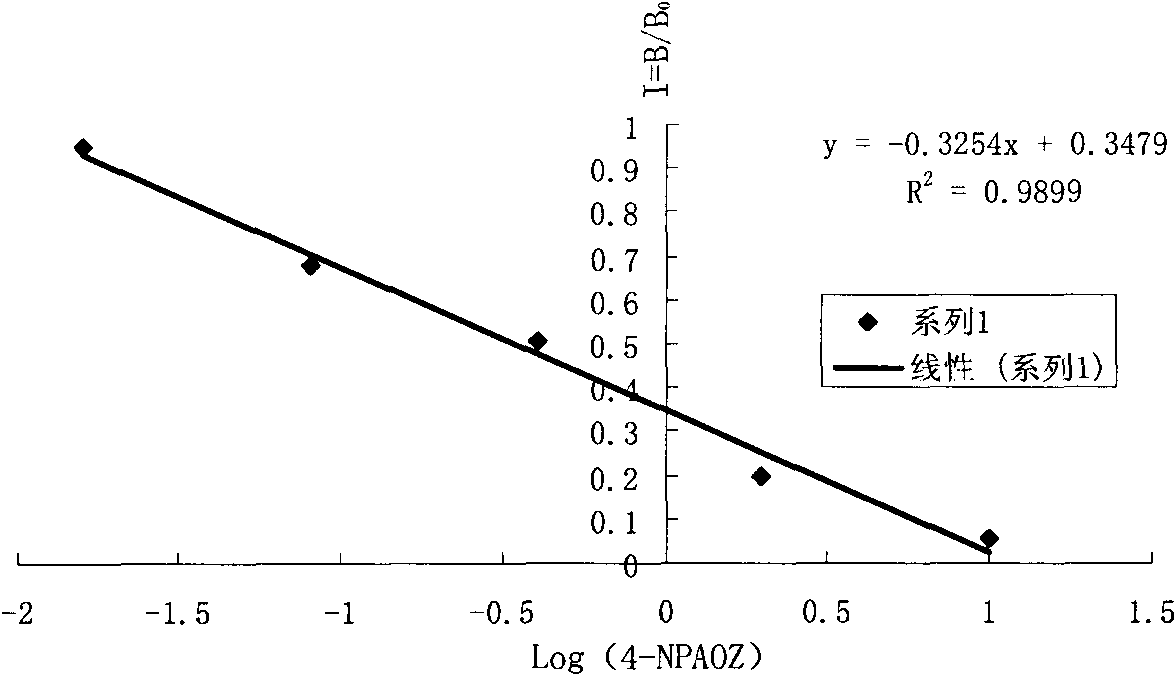
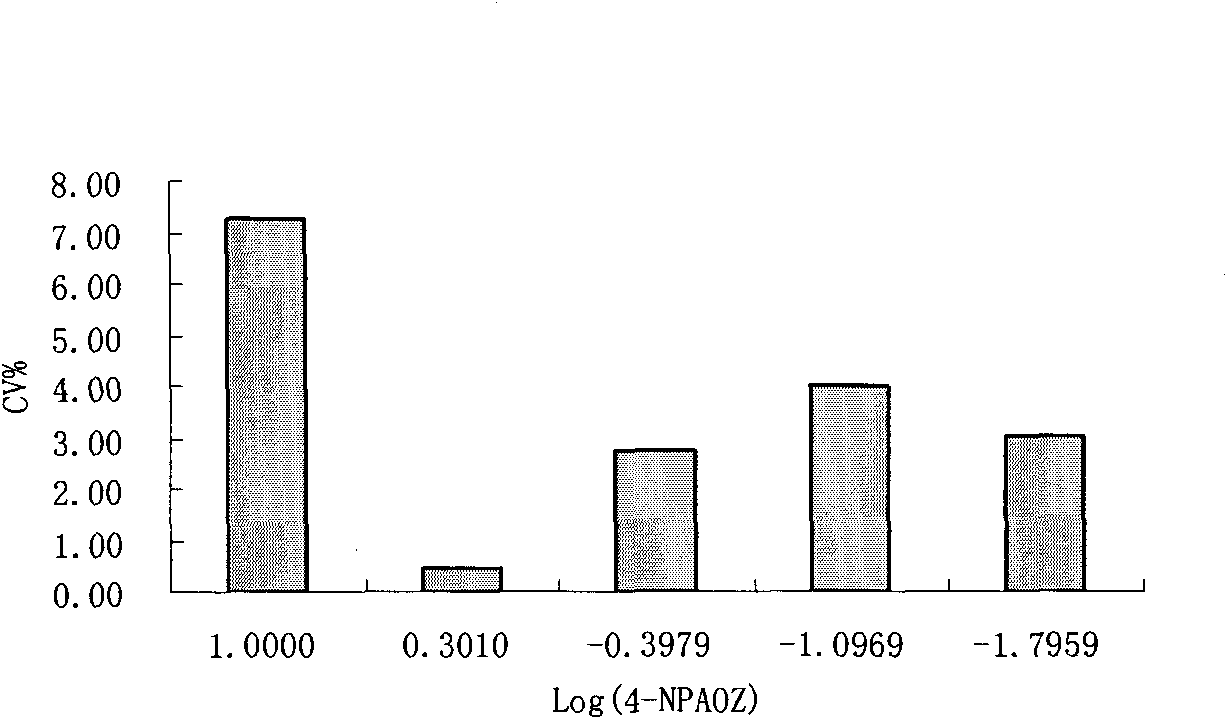
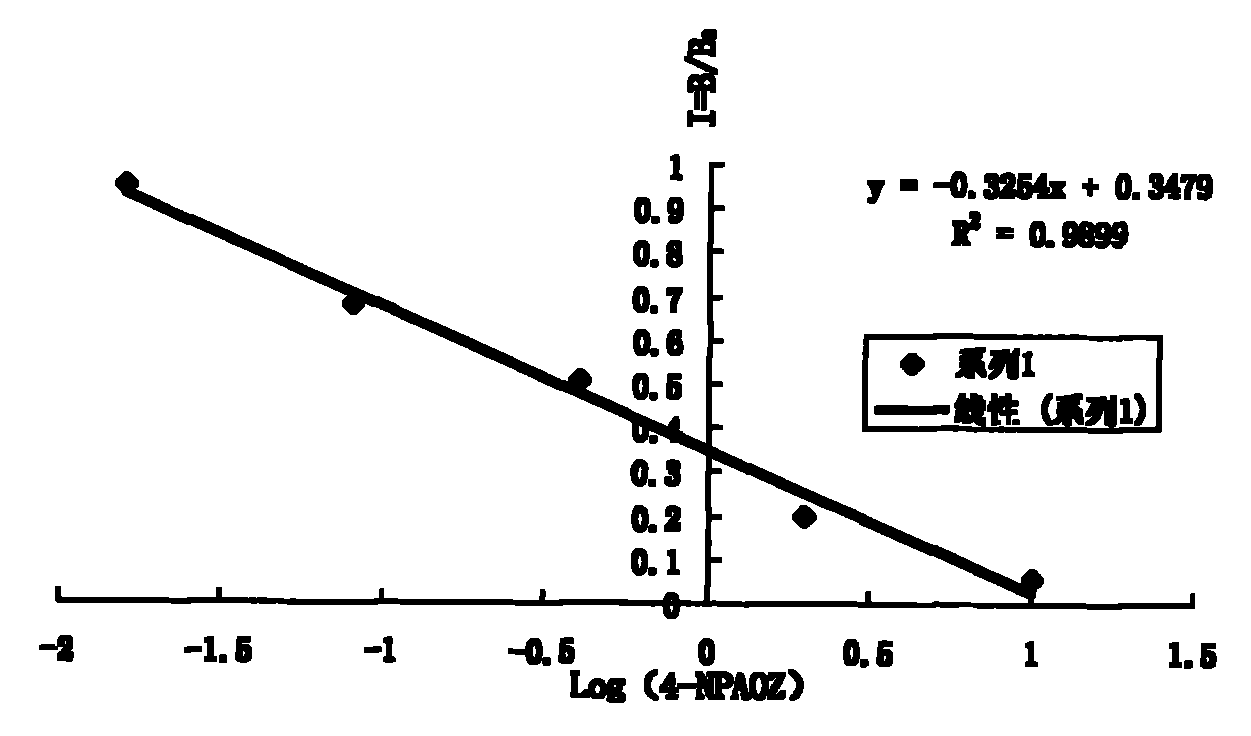Detection kit and method of furazolidone metabolin
A technology for furazolidone and metabolites, which is applied in the field of furazolidone metabolite detection kits, can solve the problems that the sensitivity of banned drugs cannot meet the requirements, is not suitable for rapid detection, and the instrument is expensive, and achieves reduction of subjectivity, simple and fast operation, and high performance. Effects of Specificity and Sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of monoclonal antibodies
[0035] (1) Preparation of immune antigen
[0036] The furazolidone metabolite AOZ reacts with p-aldehyde benzoic acid to prepare 4-CPAOZ. Then 4-CPAOZ is coupled with bovine serum albumin to obtain 4-CPAOZ-BSA conjugate, which is the immune antigen.
[0037] (2) Monoclonal antibody preparation
[0038] 1) Animal immunization: immunize mice with the above-mentioned synthetic immune antigen, and mix the immune antigen with the same amount of Freund's complete adjuvant to make an emulsifier during the first immunization, and then subcutaneously inject multiple points on the back of the neck, taking the same at 2-3 weeks apart The dose of immune antigen plus the equal amount of Freund's incomplete adjuvant is mixed and emulsified, and the booster is once. After the fourth immunization, the abdominal cavity is boosted once, and the spleen cells are taken 3 days later.
[0039] 2) Cell fusion and cloning: Take spleen cells of immunized...
Embodiment 2
[0042] Example 2 Preparation of the detection plate and reagents in the furazolidone metabolite AOZ detection kit
[0043] In this example 2, the furazolidone metabolite AOZ detection kit is equipped with a detection plate, detection target 4-NPAOZ standard solution, AOZ derivatized hapten monoclonal antibody, enzyme-labeled secondary antibody, substrate reaction solution, derivatizing agent, The sample diluent and 10× concentrated washing solution are prepared as follows:
[0044] (1) Preparation of AOZ microwell detection plate for furazolidone metabolism
[0045] Use 0.2M carbonate buffer of pH 9.6 as coating solution, dilute furazolidone metabolite derivatized hapten and ovalbumin conjugate (4-CPAOZ-OVA) to 200ng / ml, add 100μl / well In the chemiluminescence well plate, coat overnight at 4°C. Spin dry, add 1% gelatin, pH 7.4 phosphate buffer at 37°C for 2 hours at 200μl / well, wash with 1% sodium azide, pH 7.4 phosphate washing solution 3 times, spin dry, and pack Store in a bag ...
Embodiment 3
[0056] Example 3 Using the kit of the present invention to detect furazolidone metabolite AOZ
[0057] (1) Sample pretreatment
[0058] Pretreatment of animal tissues (chickens, pigs), honey, eggs, fish, and shrimp samples:
[0059] ①Take 1g sample into a centrifuge tube, add 4ml water, and fully break it under a homogenizer;
[0060] ②Then add 0.5ml of 1M HCl, 25μl of 25mM p-nitrobenzaldehyde, vortex for 1 minute and derivatize at 37°C for 16 hours (or derivatize at 55°C for 4 hours);
[0061] ③Cool to room temperature, add 0.1mol / LNa 2 HPO 4 5mL, 1mol / L NaOH 0.4mL, shake for 1min, add ethyl acetate 5mL, shake for 1min, centrifuge at 5000r / min for 6min;
[0062] ④ Take 2.5mL of ethyl acetate layer and blow dry with nitrogen, add 1mL of n-hexane to dissolve, and add 1mL of sample diluent, shake for 1min, centrifuge at 5000r / min for 10min;
[0063] ⑤Remove the lower clear liquid and pay attention to the neutral pH value, dilute it with the sample diluent for later use.
[0064] (2) The test...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com