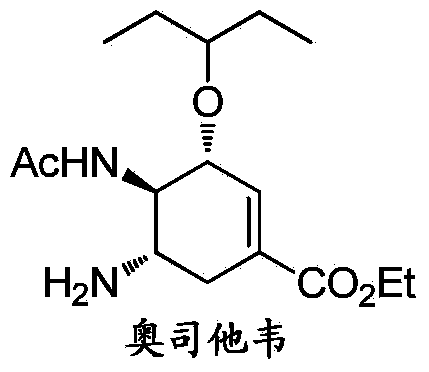
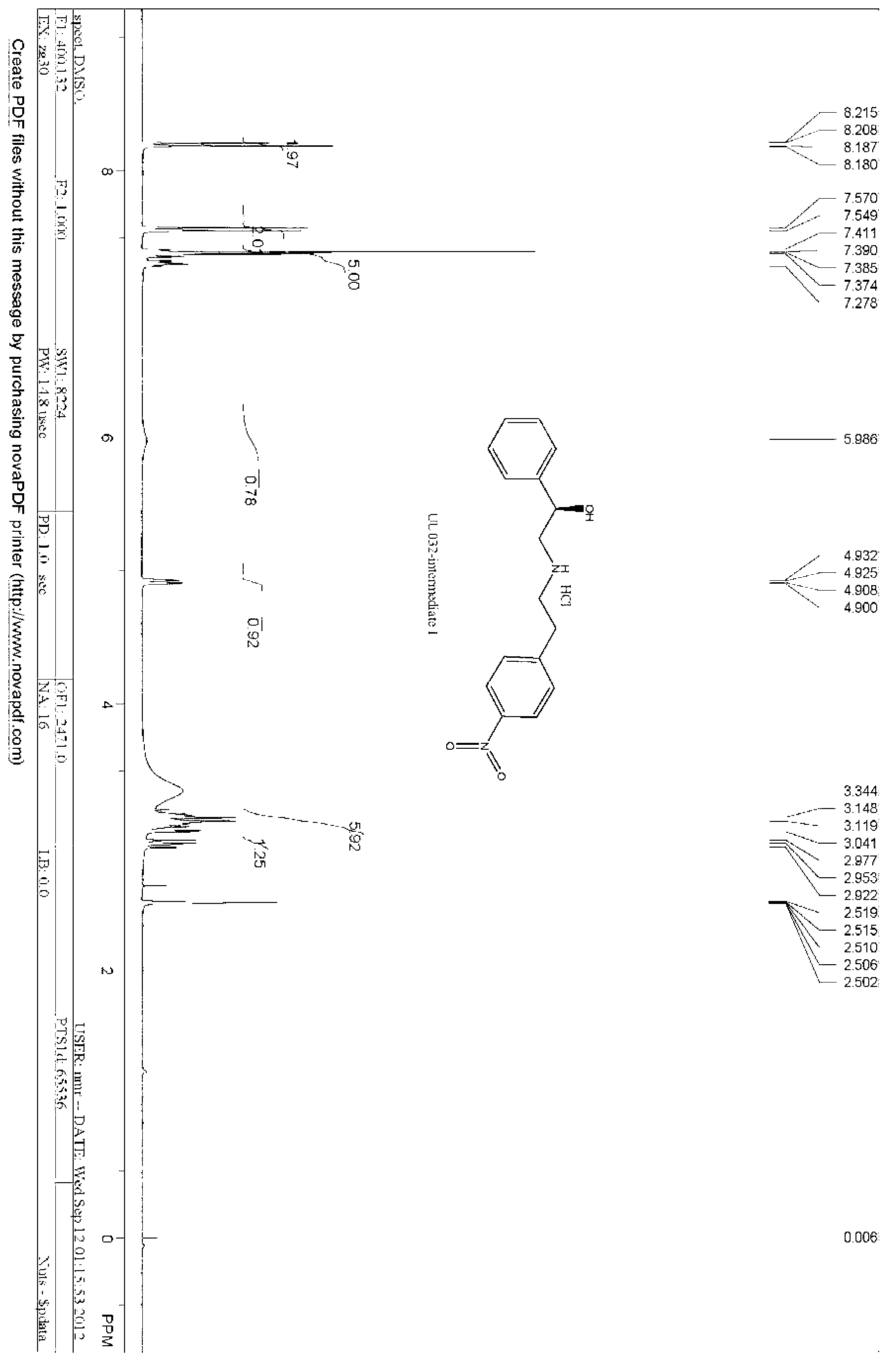
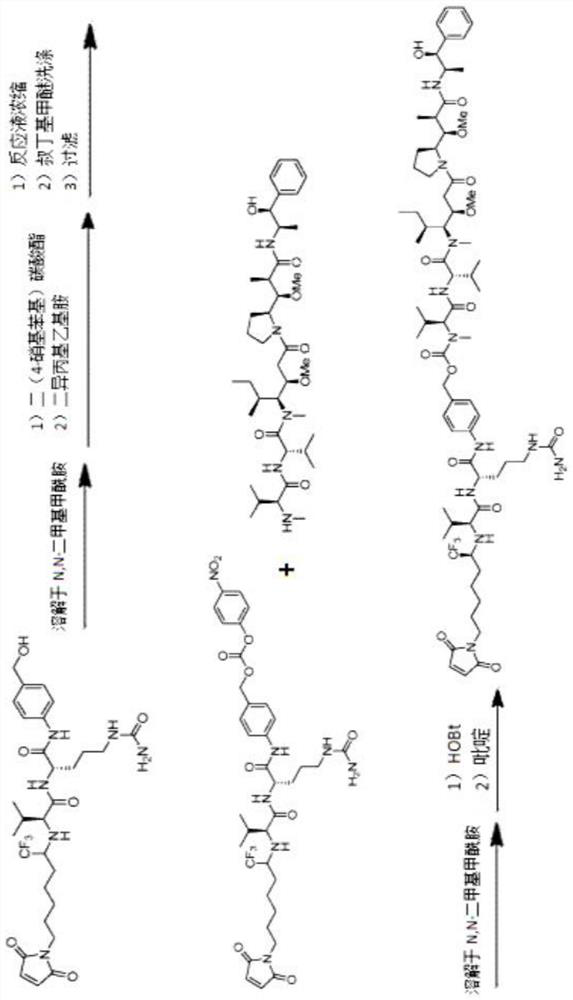
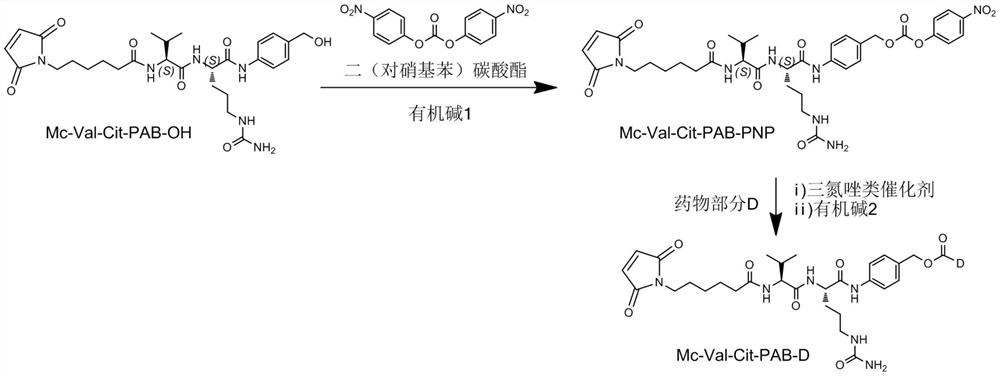
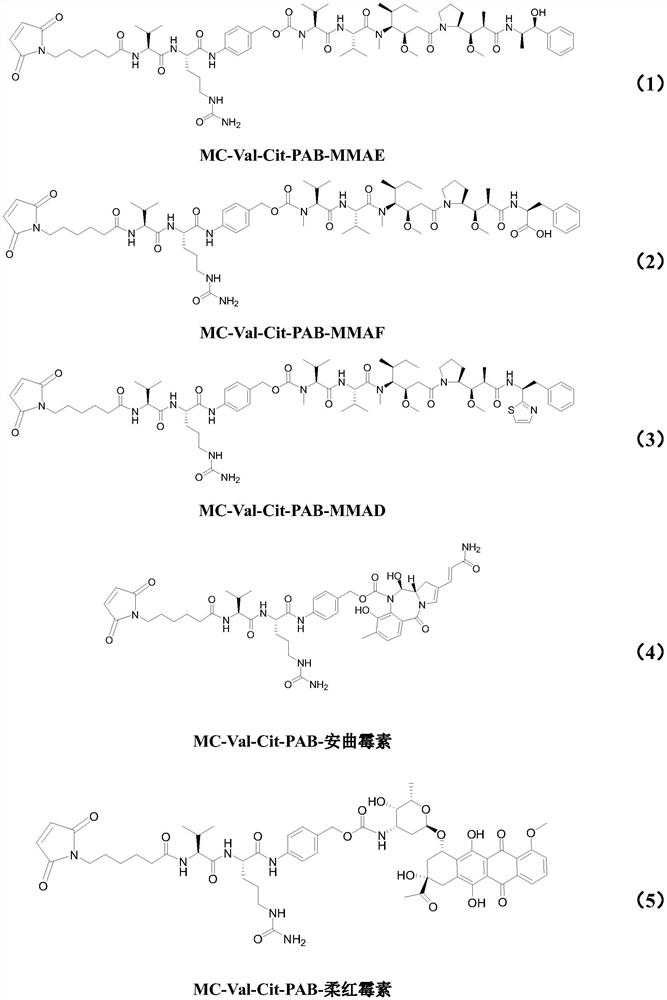
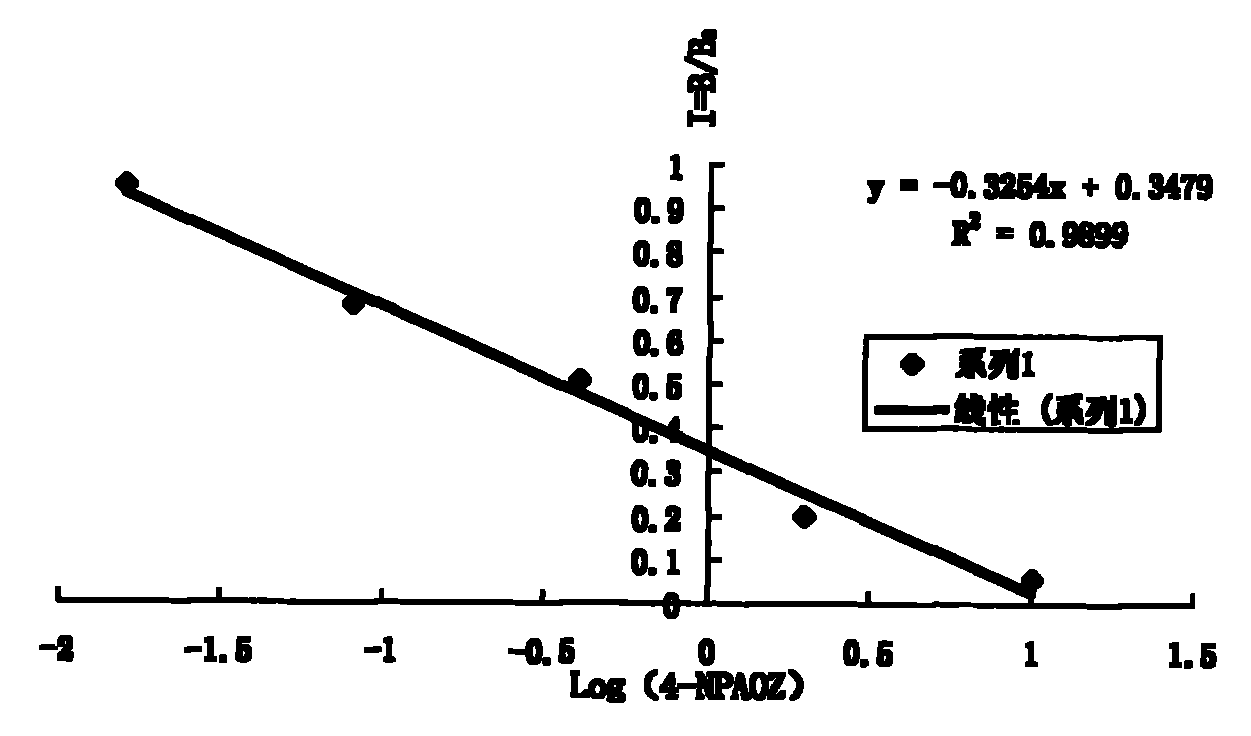Patents
Literature
170 results about "P-Nitrobenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
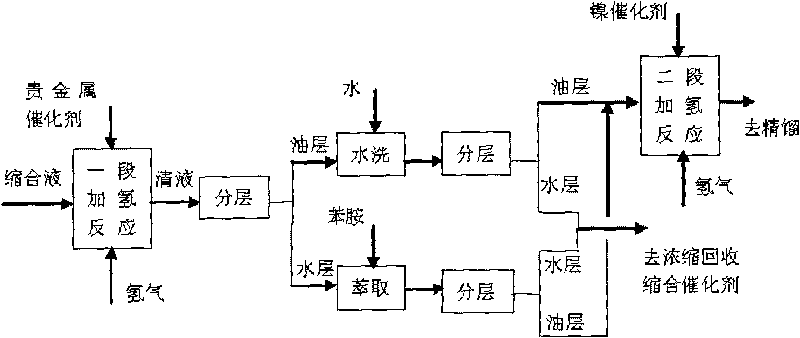
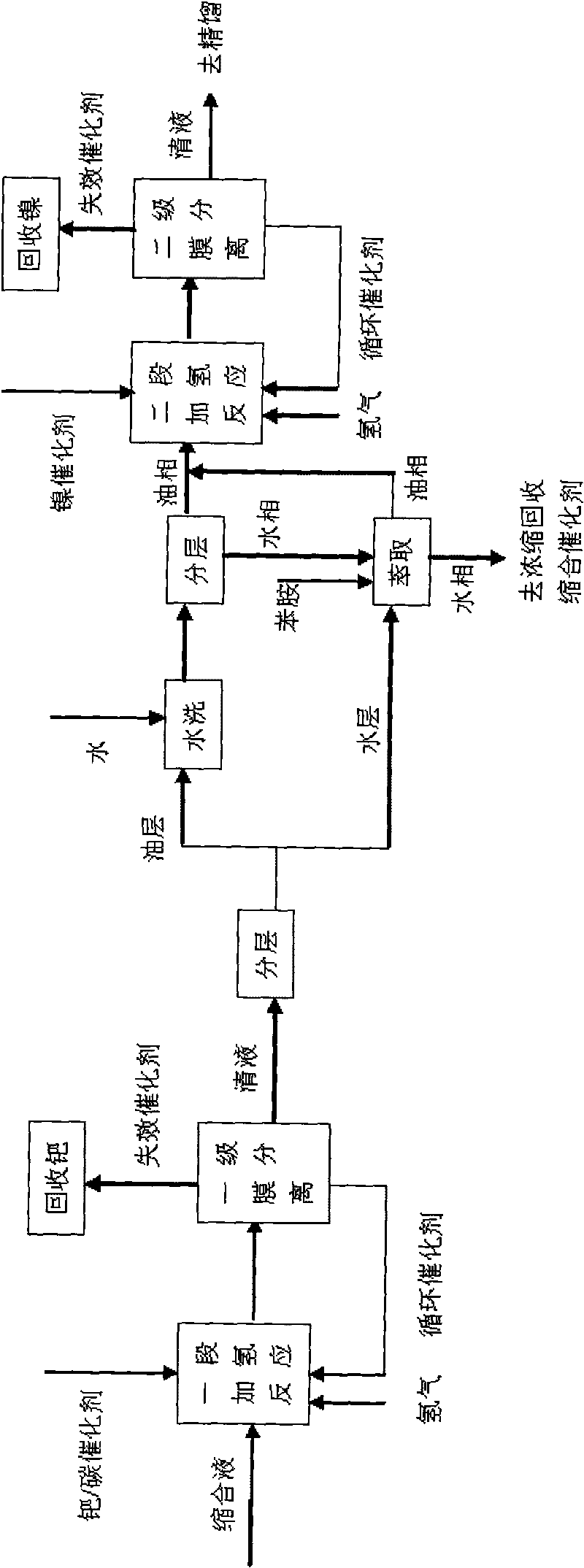
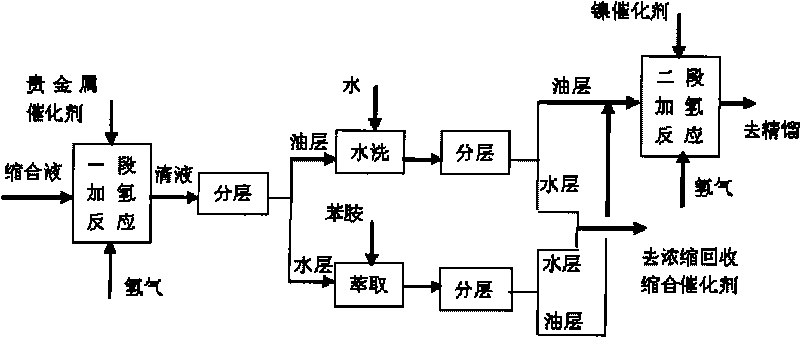
Method for preparing 4-amino diphenylamine by catalytic hydrogenation
ActiveCN101691332AEasy to cleanImprove regenerative abilitySemi-permeable membranesAmino compound purification/separationNitrosoNitrobenzene
The invention provides a method for preparing 4-amino diphenylamine by catalytic hydrogenation. The method adopts two sections of hydrogenation reaction processes, and comprises the following steps: performing the condensation reaction of condensation solution formed by the condensation reaction of nitrobenzene and aniline which serve as raw materials under an alkaline condition by using using a noble metal hydrogenation catalyst and a nickel catalyst sequentially so as to make the conversion rate of 4-nitroso diphenylamine, 4-nitro diphenylamine and azoxybenzene achieve 100 percent; and separating the noble metal hydrogenation catalyst and the nickel catalyst by using a two-stage membrane separation component system to avoid the loss of small particles of catalyst. At the same time, the method has high degree of automation and can easily realize the continuous hydrogenation process.
Owner:JIANGSU YANGNONG CHEM GROUP +1
Process for preparing aniline
ActiveUS20070203364A1Isocyanic acid derivatives preparationAmino compound purification/separationNitrobenzeneAlkali hydroxide
Crude aniline is produced by hydrogenating nitrobenzene in the presence of a catalyst. This crude aniline is then purified by means of a single-step or multi-step distillation process in which aqueous alkali metal hydroxide solution is added to the crude aniline prior to distillation and / or during distillation of the crude aniline.
Owner:COVESTRO DEUTSCHLAND AG
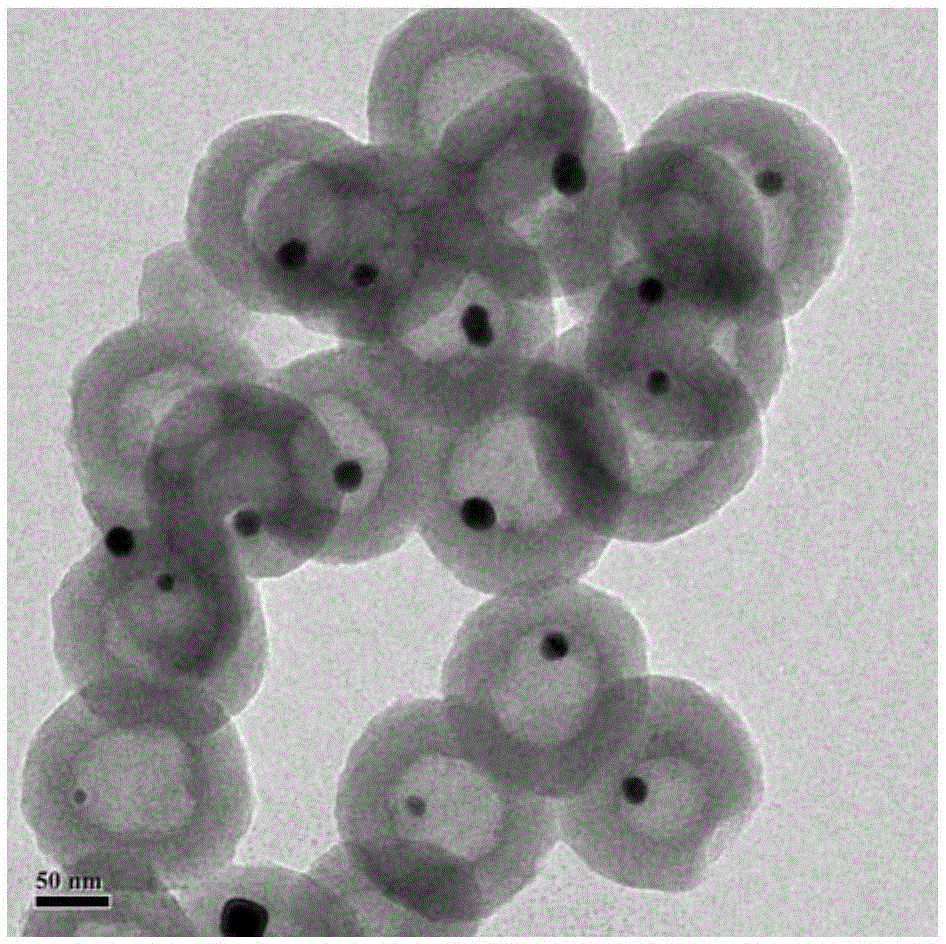
Yolk-eggshell structure Au@ hollow carbon nanosphere composite material and preparation and application thereof
The invention discloses a yolk-eggshell structure Au@ hollow carbon nanosphere composite material and preparation and application thereof. A preparation method includes the following steps that sodium citrate is adopted for reducing chlorogoldacid, and nanogold hydrosol is prepared; the nanogold hydrosol, organic monomers, water and a Triton X-100 aqueous solution are stirred and mixed; then, an initiating agent is added into a system to initiate polymerization of the monomers, and precursors are prepared; the organic monomers are composed of aniline and pyrrole; and under the inert atmosphere, the precursors are processed in a high-temperature carbonizing manner so that the yolk-eggshell structure Au@ hollow carbon nanosphere composite material can be prepared. According to the preparation method, the tedious steps that a template is prepared, complicated surface modification is needed and the template is removed are not needed, and preparation is easy and convenient. The specific surface area and the size of the prepared material can be regulated and controlled through the carbonization condition and the concentration of the organic monomers, the specific surface area is high, and the prepared material has a good catalytic effect on nitrobenzene and p-nitrophenol.
Owner:SUN YAT SEN UNIV
Nitrogen-doped porous carbon supported metal M@CN composite catalytic material, preparation method and application
InactiveCN109908940AHigh catalytic activityReduce dosagePhysical/chemical process catalystsOrganic compound preparationPorous carbonCopper
The invention discloses a nitrogen-doped porous carbon supported metal M@CN composite catalytic material, a preparation method and application, and belongs to the field of new materials. The compositecatalytic material is obtained by carbonizing a double-ligand MOF material M2 (BDC) 2 (BPY) synthesized by a nitrogen-containing organic ligand at a high temperature, wherein BDC is terephthalic acid, BPY is 4, 4'-bipyridine, and metal is one or more of copper, cobalt and nickel. The MOF material M2 (BDC) 2 (BPY) of the nitrogen-containing organic ligand is carbonized at a high temperature of 400-800 DEG C for 2-8 hours to obtain the composite catalytic material M@CN. The catalytic material is used for reduction reaction of p-nitrobenzene, and has the advantages of small catalyst use amount,mild reaction conditions and high catalytic activity.
Owner:DALIAN UNIV OF TECH
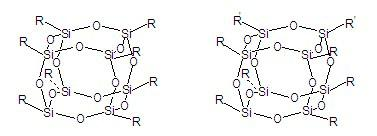
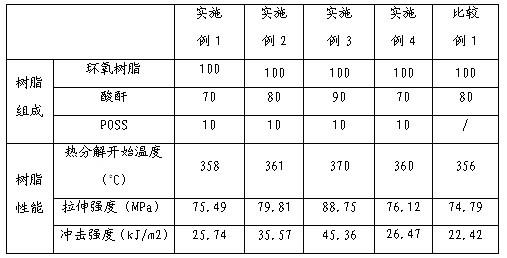
Preparation method for tough epoxy acid anhydride impregnating varnish and impregnating varnish
ActiveCN102618150AOvercome compatibilityOvercoming dispersionEpoxy resin coatingsHydrogen atomRoom temperature
The invention discloses a preparation method for touch epoxy acid anhydride impregnating varnish and impregnating varnish. The method comprises the following steps of: putting 100 parts by weight of epoxy resin into a reaction kettle; heating to 60 DEG C; adding 70-90 parts by weight of acid anhydride, and stirring at the rotating speed of 100-200 revolutions per minute for 30 minutes; adding 1-50 parts by weight of cage-shaped polyhedral oligomeric silsesquioxane (POSS); and continually stirring at the room temperature at the rotating speed of 100-200 revolutions per minute for 1 hour to form the epoxy acid anhydride impregnating varnish. The adopted cage-shaped POSS is cage-shaped POSS containing an active group or an inertial group capable of participating in an epoxy resin reaction, and the general formula can be shown as RXRY(SiO1.5)n, wherein n=8; x+y=8; and R can be one of an active aliphatic epoxy group, an aliphatic epoxy group, amino and hydrogen atom or one of inertial groups selected from phenyl, cyclopentyl, chloropropyl and p-nitrobenzene, and has superior comprehensive performance. Particularly, the toughness is improved remarkably, and the toughness of a material is improved by 10-200 percent.
Owner:ZHUZHOU TIMES ELECTRIC INSULATION
Method for treating nitrobenzene wastewater by using ozone biological aerated filter and application thereof
InactiveCN104591377AEfficient degradationImprove biodegradabilitySustainable biological treatmentBiological water/sewage treatmentBiological oxidationNitrobenzene
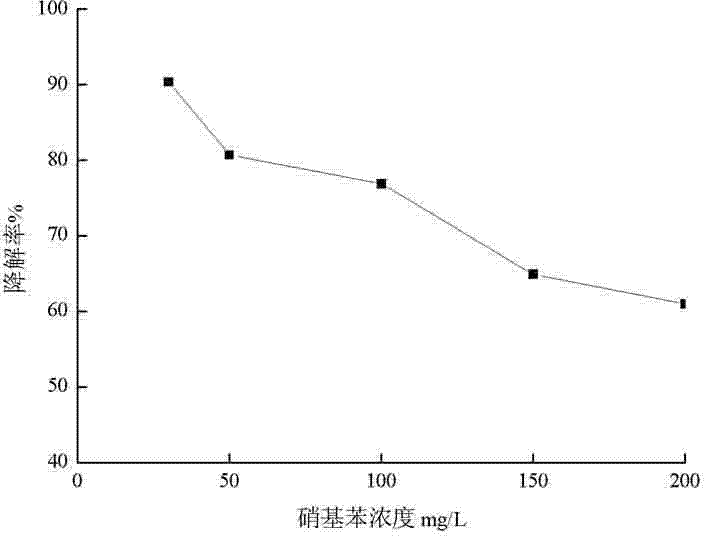
The invention discloses a method for treating nitrobenzene wastewater by using ozone biological aerated filter and application thereof and belongs to the field of sewage treatment. The treatment method comprises the following steps: (1) constructing a biological filter; (2) starting an air aerated biological filter; (3) starting an ozone biological aerated filter; (4) running the ozone biological aerated filter. The catalytic oxidation and the biological oxidation of ozone zeolite are integrated, the strong oxidative of the ozone is used for opening the ring of the nitrobenzene, the nitrobenzene after ring opening is used as an easily degradable matrix to participate in co-metabolism degradation of nitrobenzene, the removal effect of the nitrobenzene is improved, the biological film growing on the zeolite filler at the upper part of a reactor is used for degrading oxidation products, and the efficient degradation of the nitrobenzene is realized in the same reactor. Through the adoption of the biological aerated filter disclosed by the invention, removable efficiency of the nitrobenzene can reach 90% or above; the biological aerated filter has the advantages of small floor area, high nitrobenzene removal efficiency, convenient starting and simple operation.
Owner:NANJING UNIV
Method for preparing aniline through nitrobenzene hydrogenation and preparation method for catalyst used in method
ActiveCN111085241ANovel structureSmall particle sizeMolecular sieve catalystsOrganic compound preparationMolecular sievePtru catalyst
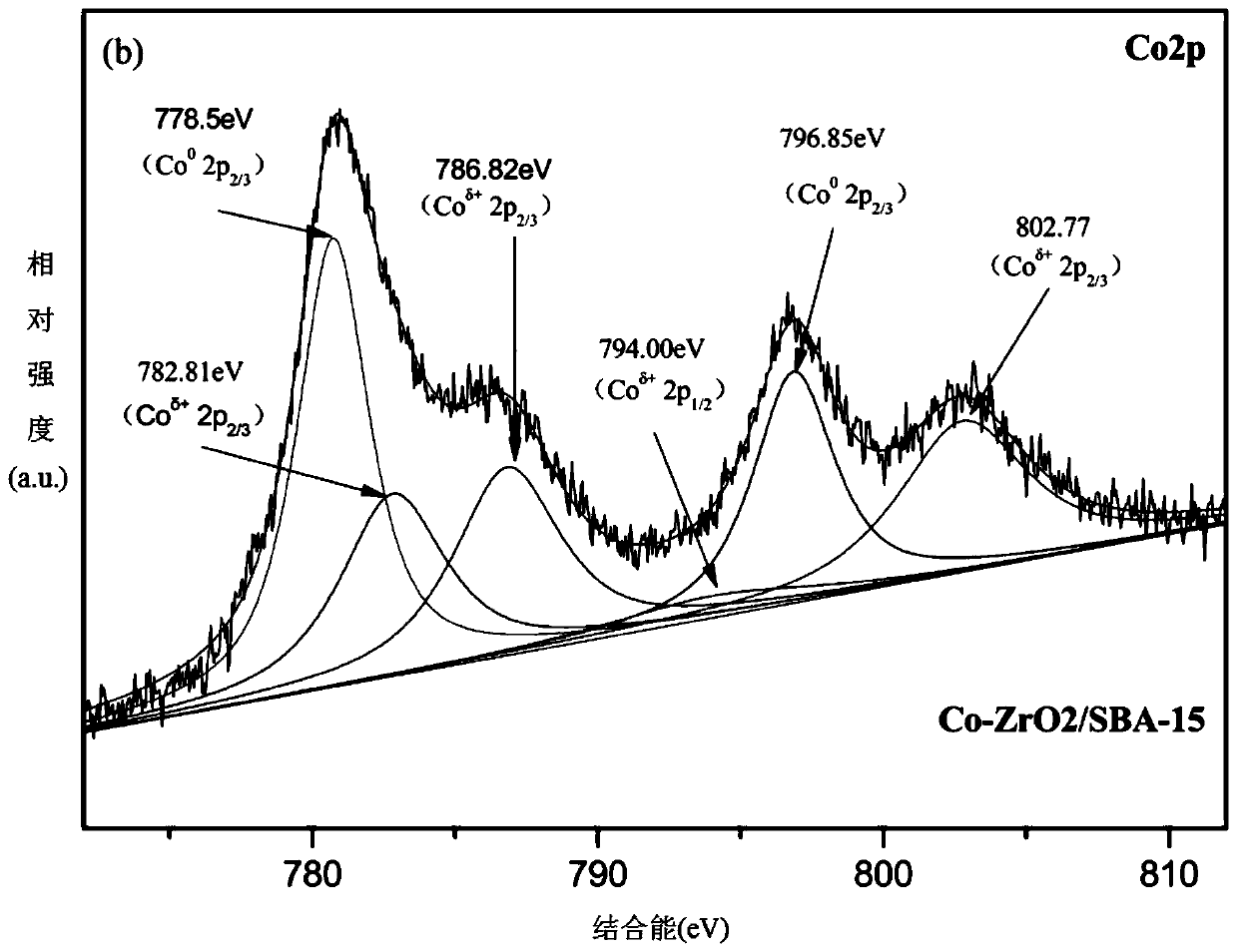
The invention provides a method for preparing aniline through nitrobenzene hydrogenation. The method comprises the step of adopting a catalyst loaded with cobalt on a composite carrier for catalytic reaction, wherein the composite carrier is a composite carrier forming a zirconium oxide structure on an SBA-15 molecular sieve; and the catalyst is Co-zirconium oxide / SBA-15. The invention also provides a preparation method for the catalyst. The preparation method comprises the following steps: preparing the composite carrier, then impregnating the composite carrier, and loading cobalt onto the composite carrier in a hydrogen environment by using a high-temperature gas-phase reduction method so as to obtain the catalyst. The catalyst for preparing the aniline through nitrobenzene hydrogenation, which is prepared by adopting a gas phase reduction method, has the advantages of high cobalt nanoparticle dispersity, small particle size, large carrier specific surface area and novel catalyst structure. The catalyst is used for selective hydrogenation of nitrobenzene; reaction conditions are mild; catalytic activity is good; and high selectivity (more than 99%) and conversion rate (more than99%) can be simultaneously achieved in the reaction of preparing the aniline through nitrobenzene hydrogenation.
Owner:XIANGTAN UNIV
Preparation and application of long-chain p-nitrobenzoylhydrazone gelator and organic metal gel thereof
InactiveCN105837469AObvious color changeImprove stabilityHydrazone preparationFluorescence/phosphorescenceHydrogenBinding site
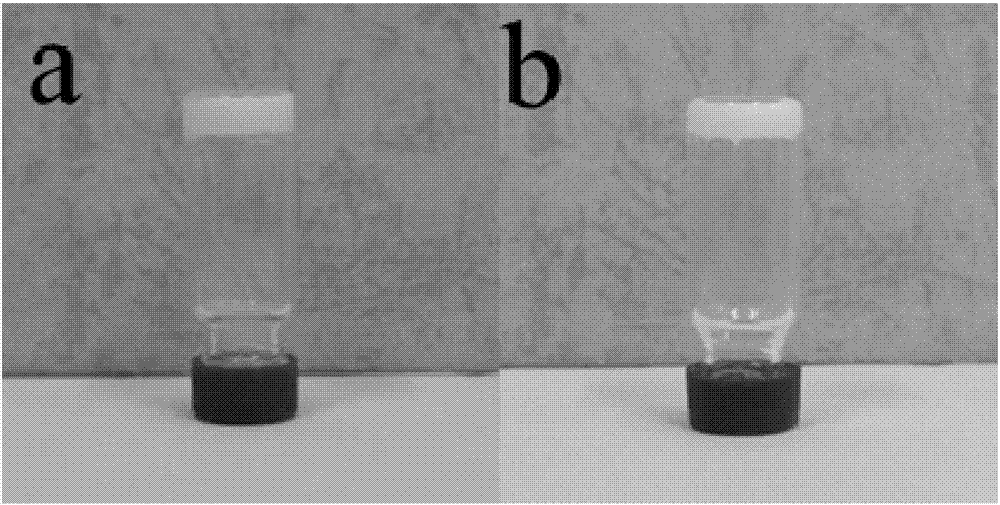
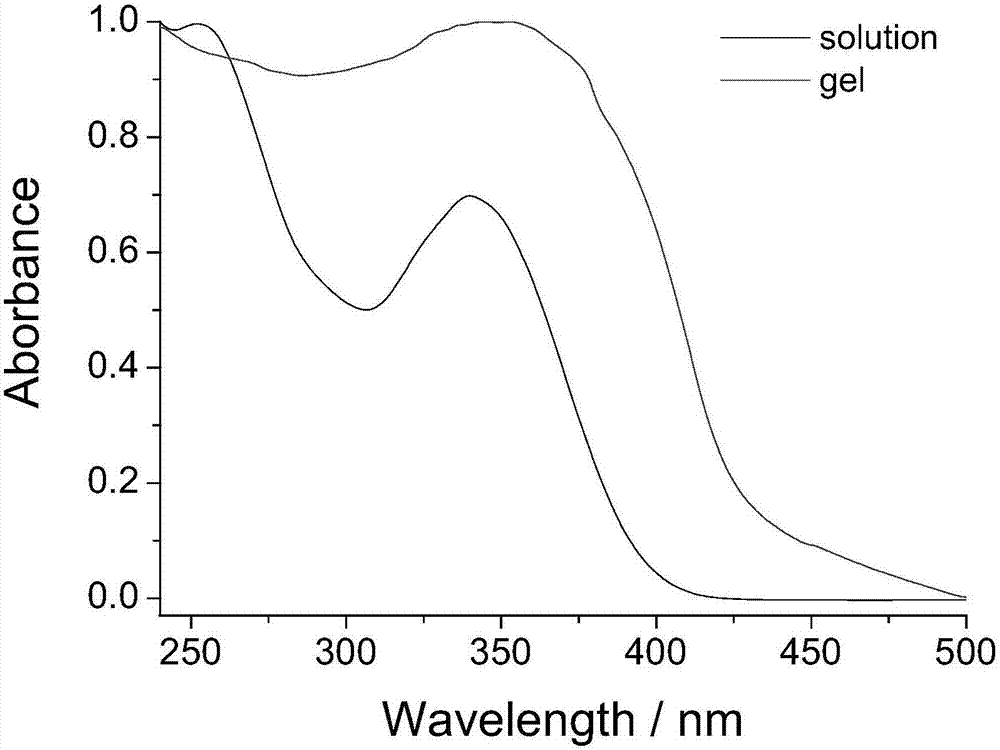
The invention provides a long-chain p-nitrobenzoylhydrazone gelator. One side of the gelator is three long alkyl chains which have 16 C and are obtained through etherification, and the three long alkyl chains can form initial driving force for gelation through mutual action among carbon hydrogen; middle -NH obtained through a hydrazinolysis reaction can form stable gel with -CH and C=O adjacent to -NH through action of intermolecular hydrogen bonds and can also form corresponding binding sites with metal ions through coordination so as to further form the organic metal gel; the formed organic metal gel can realize rapid highly-selective fluorescent recognition of I<-> in water and is obvious in color changes, which enables detection with the naked eyes to be conveniently and rapidly realized; and the formed organic metal gel has good stability, is easy to store and carry and convenient to use and can be used for writing and storage of confidential materials.
Owner:NORTHWEST NORMAL UNIVERSITY
Liquid single reagent for detecting alpha-L-fucosidase and preparation method thereof
InactiveCN102967571AAnti-interferenceImprove performanceColor/spectral properties measurementsNitrobenzeneChloride
The invention discloses a liquid single reagent for detecting alpha-L-fucosidase and a preparation method thereof. The liquid single reagent contains a buffer solution, ethylenediamine tetraacetic acid, NaCl, a surface active agent, a stabilizer and a P-nitrobenzene chloride alpha-L-fucus pyranoside. The stabilizer contained in the liquid single reagent disclosed by the invention can not only effectively enhance the stability of the liquid single reagent, but also effectively eliminate the interference of sample heparin by being cooperated with the surface active agent, therefore detection accuracy and sensitivity are enhanced. A stability test result indicates that after the liquid single reagent for detecting the alpha-L-fucosidase is respectively preserved for 3 days at 42 DEG C, preserved for 14 days at 37 DEG C and preserved for 18 months at 2-8 DEG C, various properties are kept stable so as to indicate that the liquid single reagent disclosed by the invention has good stability and can resist the interference of at least 100 KU / L heparin contained in a sample.
Owner:BEIJING LEADMAN BIOCHEM
2-carbonyl-3-phenylpropionic acid p-nitrobenzoyl hydrazone di-p-methyl benzyl tin complex as well as preparation method and application thereof
ActiveCN106220670AFriendly and beneficialHigh anticancer activityTin organic compoundsOrganic chemistry methodsHydrazoneStructural formula
The invention discloses a 2-carbonyl-3-phenylpropionic acid p-nitrobenzoyl hydrazone di-p-methyl benzyl tin complex of which the structural formula is shown in the description; in the formula, Ph is phenyl, and R is methyl benzyl. The invention further discloses a preparation method and application of the 2-carbonyl-3-phenylpropionic acid p-nitrobenzoyl hydrazone di-p-methyl benzyl tin complex in preparation of anti-cancer medicines.
Owner:HENGYANG NORMAL UNIV
2-oxobutyric acid p-nitrobenzoyl hydrazone dibenzyl tin complex and preparation method and application thereof
ActiveCN106380480AHas a thermally stable rangeFriendly and beneficialTin organic compoundsOrganic chemistry methodsHydrazoneStructural formula
The invention discloses a 2-oxobutyric acid p-nitrobenzoyl hydrazone dibenzyl tin complex, which is a complex having the structural formula (I) as shown in the specification. In the structural formula (I), R is phenyl group. The invention also discloses a preparation method of the 2-oxobutyric acid p-nitrobenzoyl hydrazone dibenzyl tin complex and application of the complex in the preparation of an anti-cancer drug.
Owner:HENGYANG NORMAL UNIV
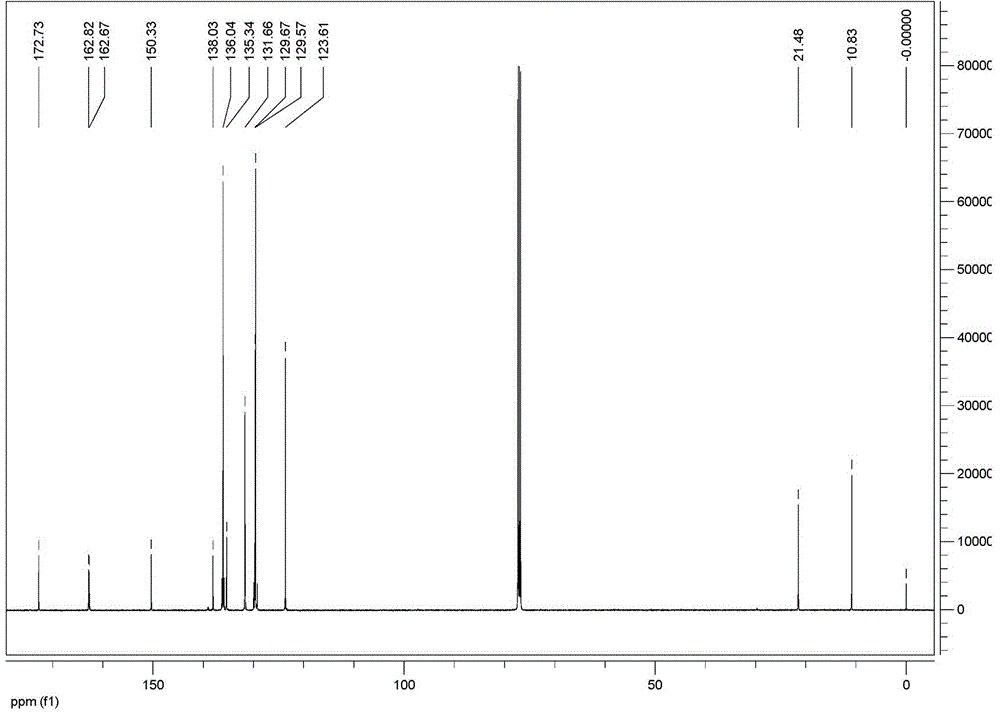
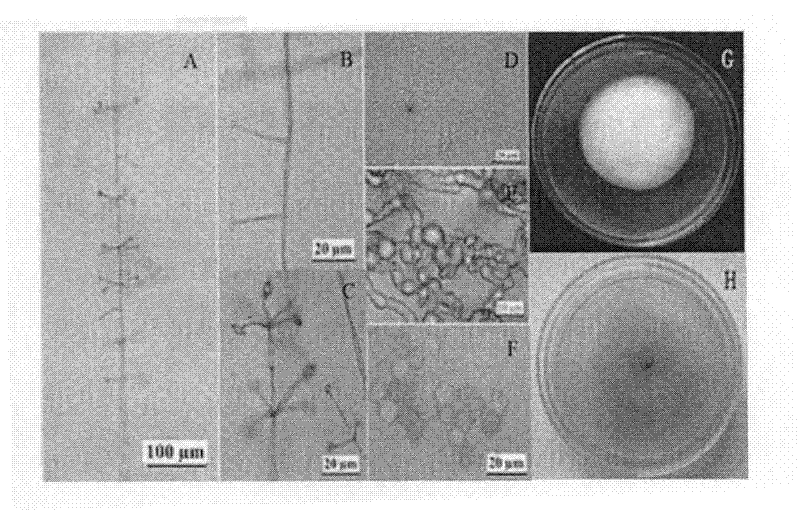
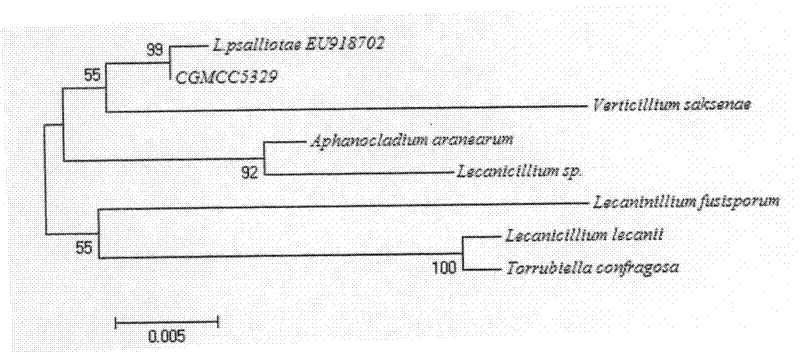
Lecanicillium psalliotae strain
InactiveCN102417886AIncrease enzyme activityIncreased parasitic abilityBiocideFungiDegradative enzymeLife stage
The invention discloses a lecanicillium psalliotae strain with the preservation number of CGMCC NO: 5329. The strain can colonise spawns, two-year-old larvae and female adults at different life stages of rootknot nematode. Activations of chitinous substance degradative enzyme and excisionenzyme generated by the strain can be respectively determined through an N-acetyl glucosamine method and a p-nitrobenzene method, the enzymatic activities thereof can reach 3.98 U / h.mL and 0.38 U / h.mL, and higher activity of the produced chitinous substance enzyme can be detected at different temperature (27 DEG C-75 DEG C) and different pH (3.0-7.0); and the influence of chitinous substance enzyme extract produced by the strain on eclosion of rootknot nematode spawns is determined in vitro, and the inhibition ratio and the destruction ratio of enzyme solution on relative eclosion of the spawns are respectively 94.52 percent and 84.32 percent. The lecanicillium psalliotae strain disclosed by the invention is applied to biological control of crop rootknot nematodiasis, provides an effective way for protecting sweet potatoes, fruits, vegetables in north China and particularly controlling destructiverootknot nematodiasis, and has obvious ecological benefit, economic value and social value.
Owner:QINGDAO AGRI UNIV
Detection kit and method of furazolidone metabolin
InactiveCN101806796AImprove featuresIncreased sensitivityChemiluminescene/bioluminescenceBiological testing3-amino-2-oxazolidoneP-Nitrobenzene
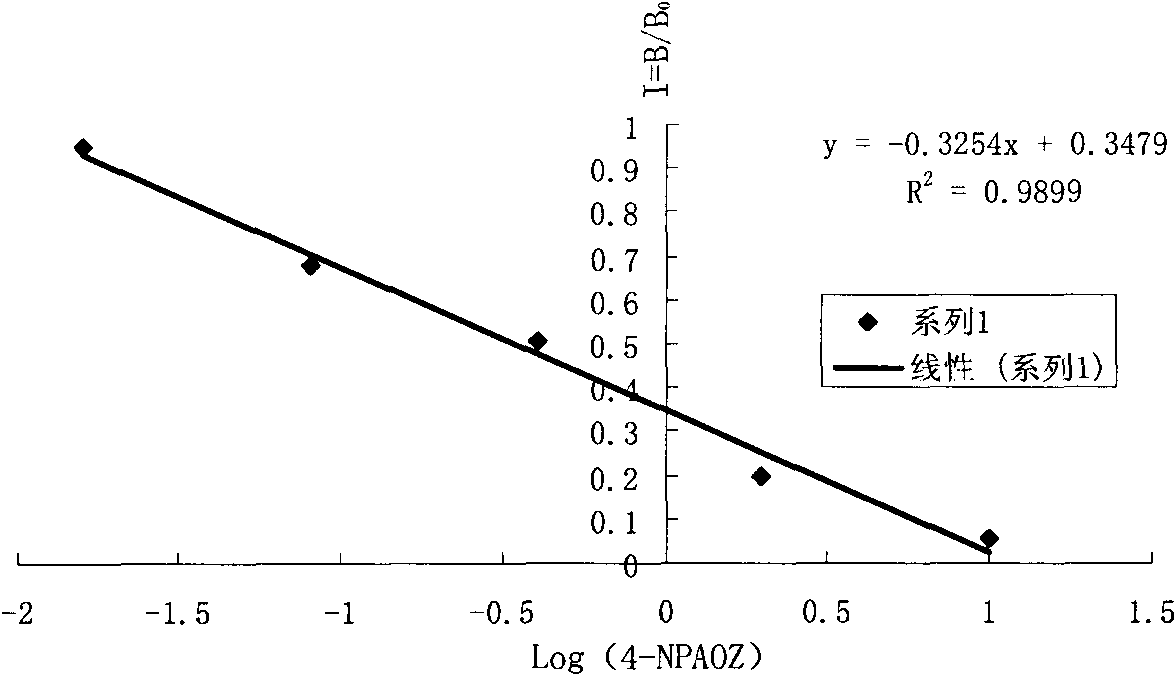
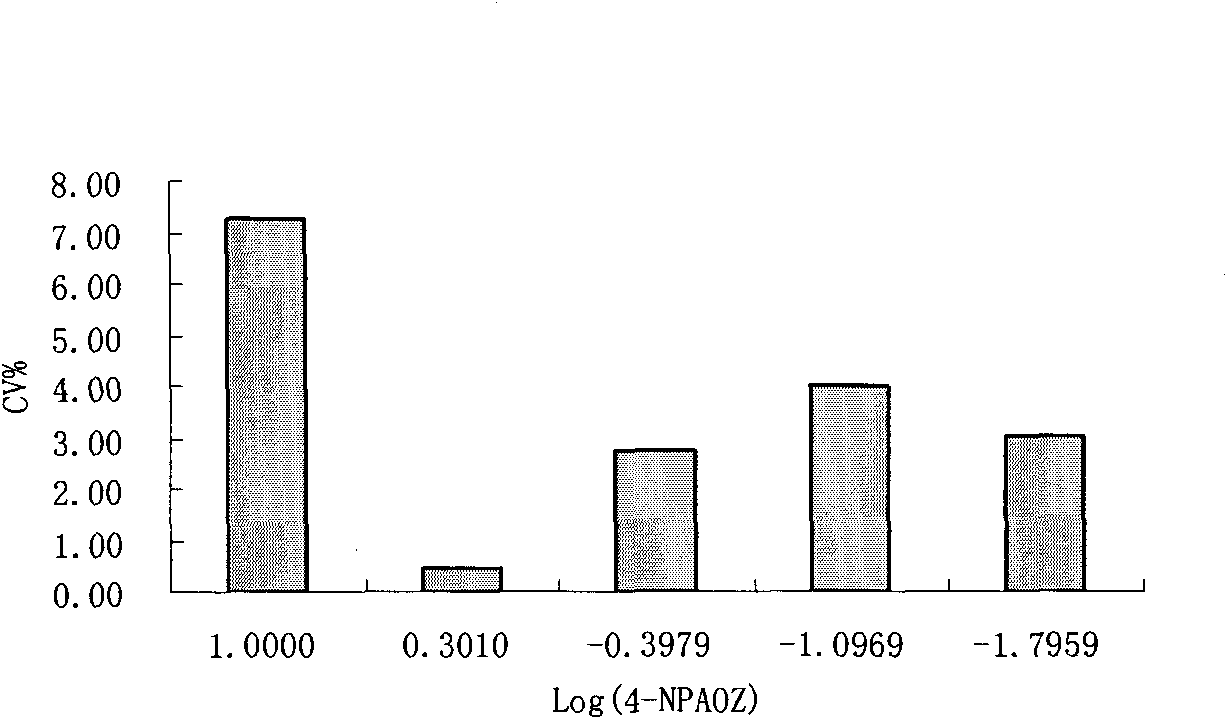
The invention discloses a chemiluminescence detection kit of furazolidone metabolin, comprising a detection plate and a reagent, wherein the reagent comprises an antibody and a detection target standard solution, wherein the detection target is a p-nitrobenzene derivative 4-NPAOZ of furazolidone metabolin 3-amino-2-oxazolidone. The detection kit of the furazolidone metabolin not only can realize the fast and mass detection, but also has very high specificity and sensitivity, and achieves the detection sensitivity of 0.005 ppb.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI +2
Method for degrading nitrobenzene in waste water
InactiveCN102730840AIncrease costCutting costsWater contaminantsBiological water/sewage treatmentResistant bacteriaEnvironmental engineering
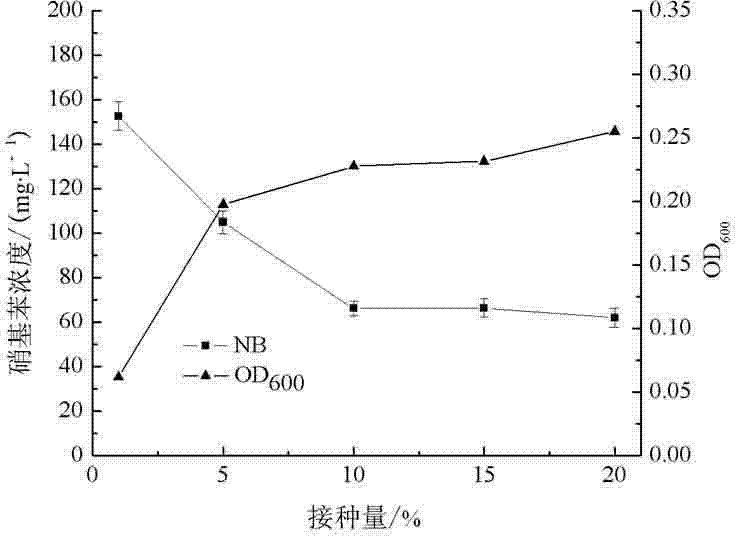
The invention relates to a method for degrading nitrobenzene in waste water, characterized by inoculating nitrobenzene low temperature-resistant bacteria in logarithmic growth phase to the waste water containing nitrobenzene according to a volume percent of 1-20%, regulating the pH value to 4-9, and culturing for 48 h under the conditions of vibration, wherein the nitrobenzene low temperature-resistant bacteria are acinetobacter baumannii, preferably acinetobacter baumannii strain cc-2. According to the invention, nitrobenzene can be effectively degraded in a low temperature environment, the defects of restraint of growth and metabolism of the strain in low temperature and insufficient capability of degrading nitrobenzene are made up, the capacity of the strain for degrading nitrobenzene in low temperature is raised, and the method provides the treatment of nitrobenzene in low temperature with a technology support.
Owner:LIAONING UNIVERSITY
Organogel compound of 4-nitrophenylthiourea, and preparation method, gel and applications of organogel compound
InactiveCN107056666AAchieving Sensitive DetectionOrganic chemistryMaterial analysis by observing effect on chemical indicatorThioureaNitrobenzene
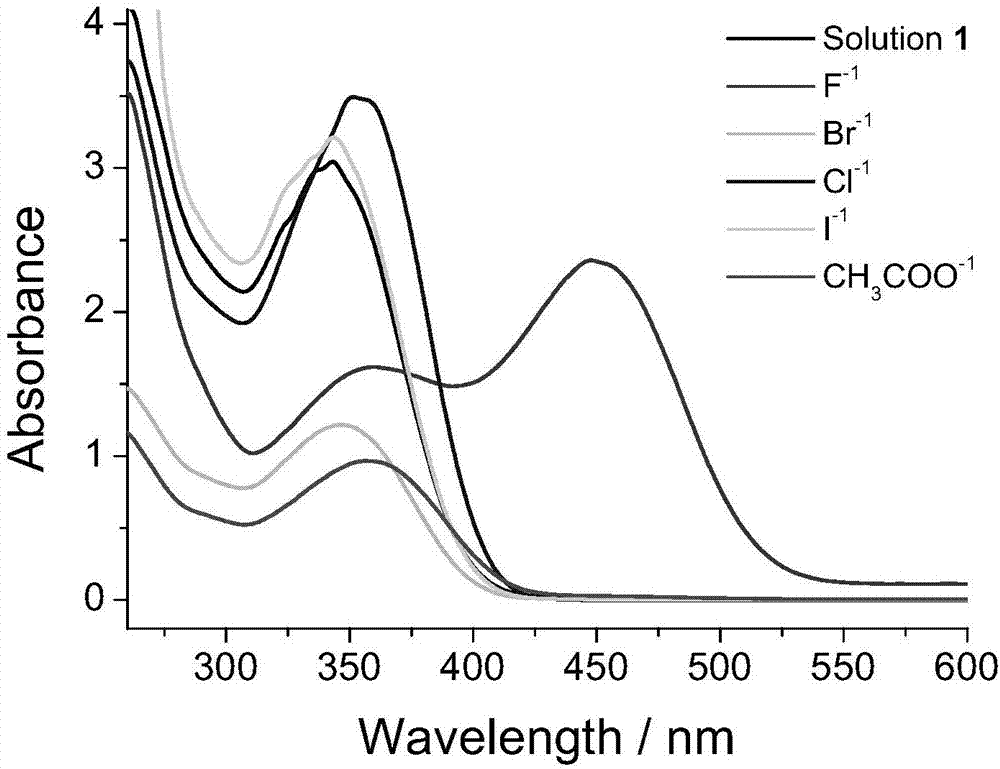
The invention discloses an organogel compound of 4-nitrophenylthiourea. The compound is 1-(2-(3,4,5-tri(dodecyl) benzamido)ethyl)-3-(4-nitrobenzene)thiourea taking (p-nitrophenyl)thiourea as a chromophore, the structural formula is as shown in the specification, and the molecular formula is respectively [4-NO2C6H4N2H2S-C2H4NHCOC6H2-(OC12H25)3]. The gel factor has the function of sensitively detecting fluorine ions and mercury ions, the color of the solution state of the gel factor is changed by adding the fluorine ions, and ring closing reaction is generated and the color of the solution state of the gel factor is changed again by adding the mercury ions; and the gel system has sensitive effects on the fluorine ions and mercury ions in a good state. By utilizing the property, the sensitive detection of the gel system on the fluorine ions and mercury ions can be realized.
Owner:XINYANG NORMAL UNIVERSITY
Preparation method and application for high-activity hydrogenation reaction catalyst
ActiveCN108855212AHigh catalytic activityRaise the ratioOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMicrosphereNitrobenzene
The invention discloses a preparation method for a high-activity hydrogenation reaction catalyst, and relates to the field of preparation methods for chemical materials. According to the preparation method, polystyrene microspheres subjected to surface functionalization serve as a matrix, the surfaces of the polystyrene microspheres are modified with palladium nanoparticles so as to obtain the catalyst of a palladium nanoparticle@PS core-shell structure, the catalyst can be used for carrying out catalytic hydrogenation reaction on nitrobenzene to prepare aniline, can be stably stored in the air and can be easily separated from reactants, the catalytic efficiency is high, the preparation process is simple, and the repeatability is high.
Owner:YANGZHOU UNIV
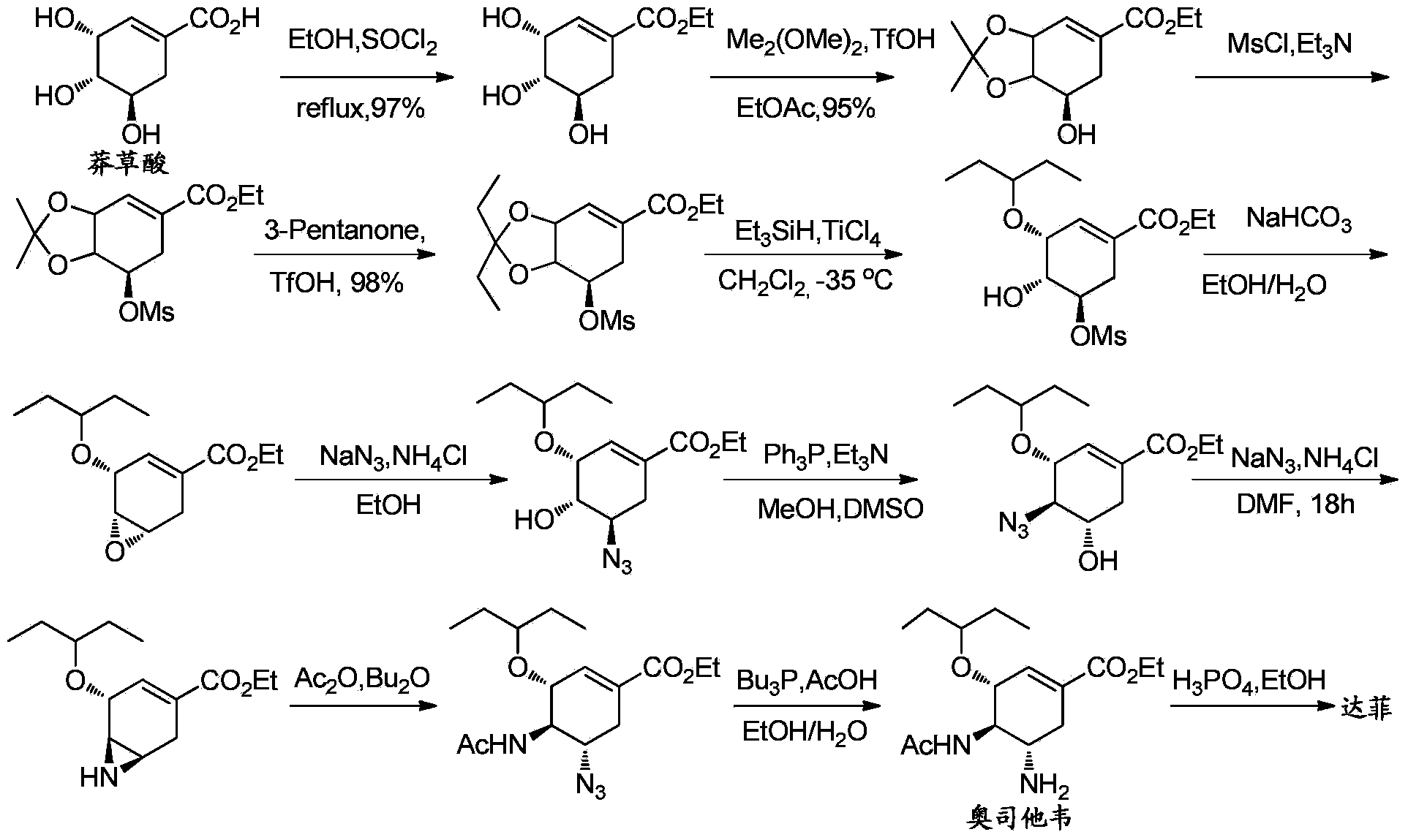
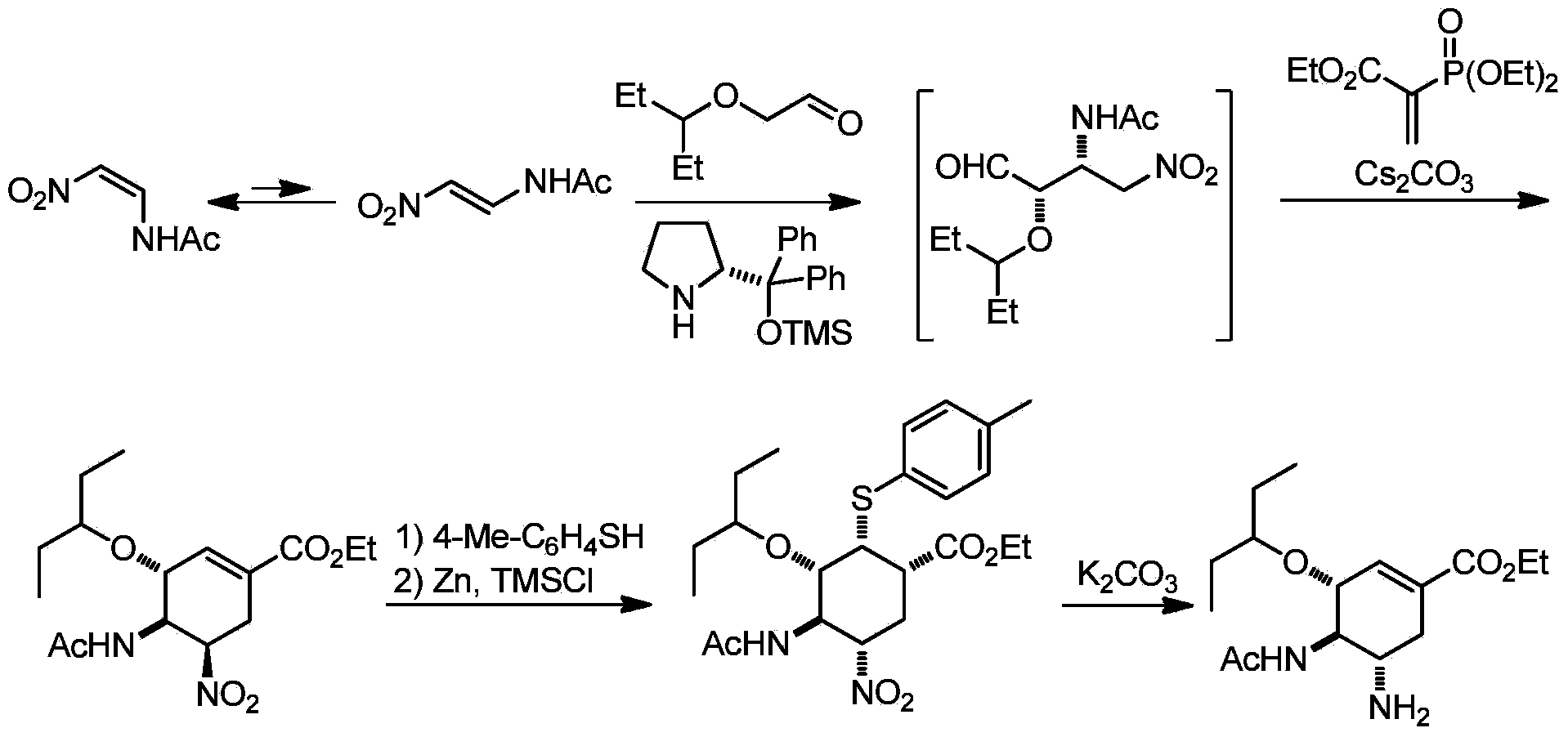
Synthesis method of oseltamivir
InactiveCN103833570AHigh yieldEasy to operateOrganic compound preparationCarboxylic acid amides preparationBird fluSynthesis methods
The invention discloses a synthesis method of oseltamivir. The synthesis method of oseltamivir comprises the following steps: starting from a compound 1,3-butadiene-3-amyl ether and compound 3-nitro-ethyl acrylate, carrying out Diels-Alder reaction, then reacting at room temperature in acetonitrile in the presence of a copper catalyst and PhI-NNs to prepare an aziridine compound in a one-pot method, wherein the mole ratio of the 1,3-butadiene-3-amyl ether to 3-nitro-ethyl acrylate to the copper catalyst is 1.1: 1: 0.025-0.1; and finally synthesizing the oseltamivir for preventing bird flu through the aziridine ring opening, nitryl and p-nitrobenzene sulfonyl removal, acetylation and hydrogenation. The method comprises short steps, the used reagent is cheap and easily available, the operation is simple, the total yield is up to 40%, and the method is a simple and efficient synthesis method of oseltamivir.
Owner:ZHEJIANG NORMAL UNIVERSITY
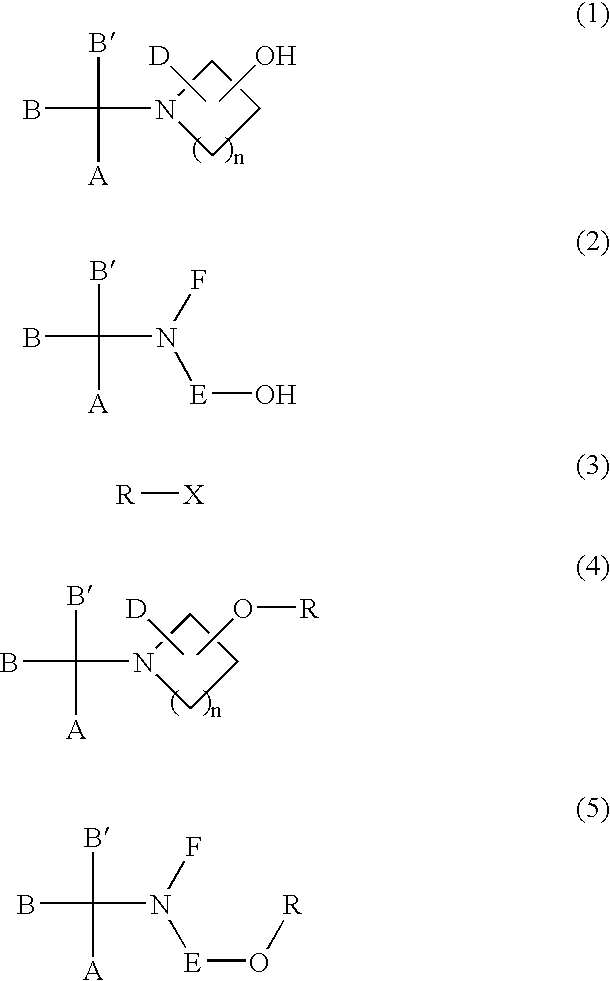
Process for the production of sulfonic esters
InactiveUS20030162966A1High reaction yieldImprove purification effectOrganic compound preparationSulfonic acid esters preparationSulfonateOrganic solvent
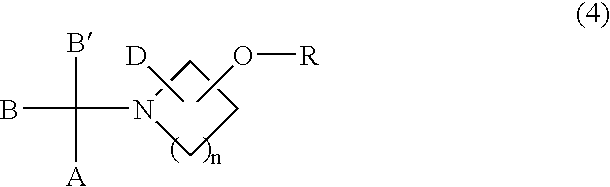
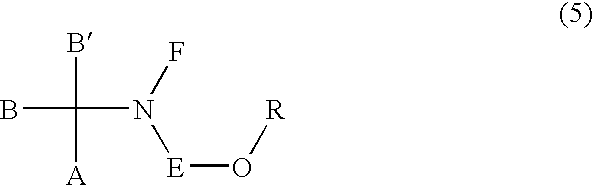
Sulfonic acid ester derivatives represented by the general formula (4) or (5) are produced by reacting an amino alcohol derivative represented by the general formula (1) or (2) with an organic sulfonyl halide represented by the general formula (3), in a mixed solvent composed of an aprotic organic solvent and water in the presence of a non-water-prohibiting inorganic base. This procedure can be carried out in a simple, easy, safe and economical manner while reducing the load on the environment. Wherein n represents an integer of 0 to 5, A represents a phenyl group, which may be substituted, R represents a methanesulfonyl, ethanesulfonyl, p-toluenesulfonyl or p-nitrobenzenesulfonyl group and X represents a chloride, bromine or iodine atom.
Owner:KANEKA CORP
2-amino phenol 1, 6-dioxygenase, its gene and use thereof
The present invention relates to one p-nitrobenzene chloride ring opening enzyme gene and its expression product. The gene is from Comamonas testosteroni CNB1 CGMCC No. 1028 strain, has size of 1770 bp, and contains two ORF opening read frames in the same direction of 942 bp and 813 bp separately, separated by 15 bp, coding two different protein products beta-subunit and alpha-subunit. Two beta-subunits and two alpha-subunits constitute one active enzyme molecule. The enzyme can catalyze the ring opening cracking in the 1 and the 6 place of 2-amino phenol and the direct ring opening cracking of p-nitrobenzene chloride. Therefore, the gene may be used in constituting gene engineering bacterian and produce active enzyme for treating sewage containing p-nitrobenzene chloride and similar compound and in biological conversion engineering.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
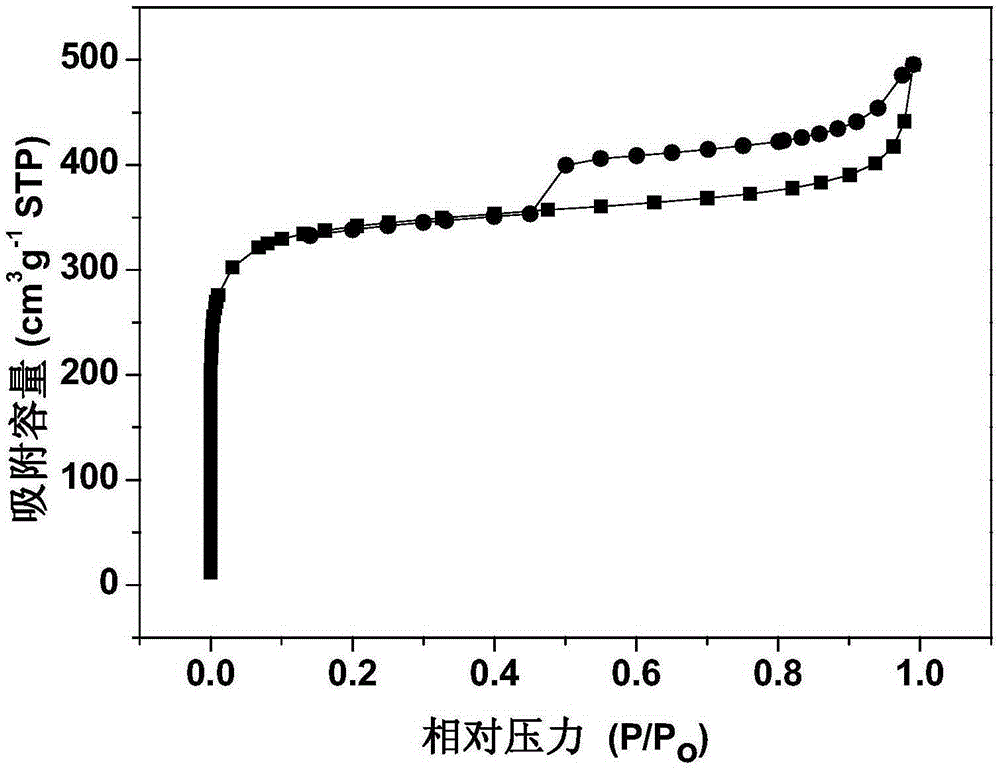
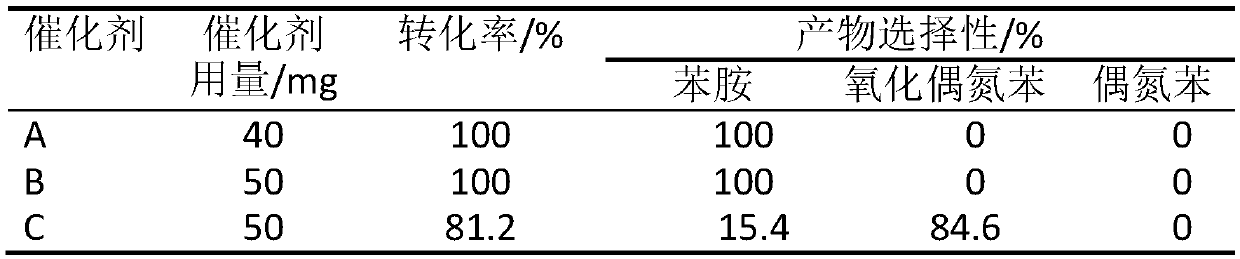
Supported yolk-eggshell structure nano-catalyst and preparation method thereof
PendingCN111215147AIncrease contact areaMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNano catalystYolk
The invention provides a supported yolk-eggshell structure nano-catalyst. The supported yolk-eggshell structure nano-catalyst comprises a carrier and an active component; the carrier is a metal organic framework; the active component is double-transition metal nanoparticles with a plasma resonance effect; and the double-transition metal nanoparticles are embedded into the carrier, so that the active component and the carrier form a yolk-eggshell structure. Compared with like catalysts in the prior art, the catalyst provided by the invention absorbs visible light and converts light energy intoheat energy by utilizing the plasma resonance effect of the supported double-transition metal nanoparticles, so that the reaction condition of p-nitrostyrolene hydrogenation is converted into normal temperature and normal pressure; and the unique yolk-eggshell structure of the catalyst makes more active sites exposed, and a cavity between the yolk and the eggshell can enrich a reaction substrate and increase the contact area between the substrate and the active site, so that the catalyst shows efficient catalytic activity and reaction selectivity in the hydrogenation reaction of p-nitrostyrolene, and has a good application prospect.
Owner:UNIV OF SCI & TECH OF CHINA
Preparation method of p-aminophenylethylamine
ActiveCN104892427AEasy to manufactureRaw materials are cheap and easy to getOrganic compound preparationAmino compound preparationSulfonyl chlorideOrganic synthesis
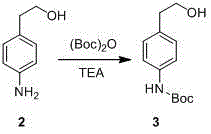
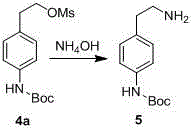
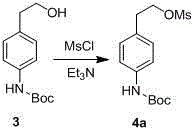
The invention discloses a preparation method of p-aminophenylethylamine, belonging to the technical field of organic synthesis. The technical scheme is as follows: the preparation method comprises the following steps: by using p-nitrophenylethanol as a raw material, carrying out catalytic hydrogenation to reduce the para-position nitro groups of the p-nitrophenylethanol into amino groups, carrying out Boc acid anhydride protection on the para-position nitro groups, carrying out substitution reaction on hydroxy groups with sulfonyl chloride compounds to obtain sulfonic acid compounds, aminating, and finally, removing Boc groups to obtain the p-aminophenylethylamine. The preparation process is simple and easy to implement, and has the advantages of cheap and accessible raw materials, higher reaction efficiency and favorable repetitiveness.
Owner:新乡市锦源化工有限公司
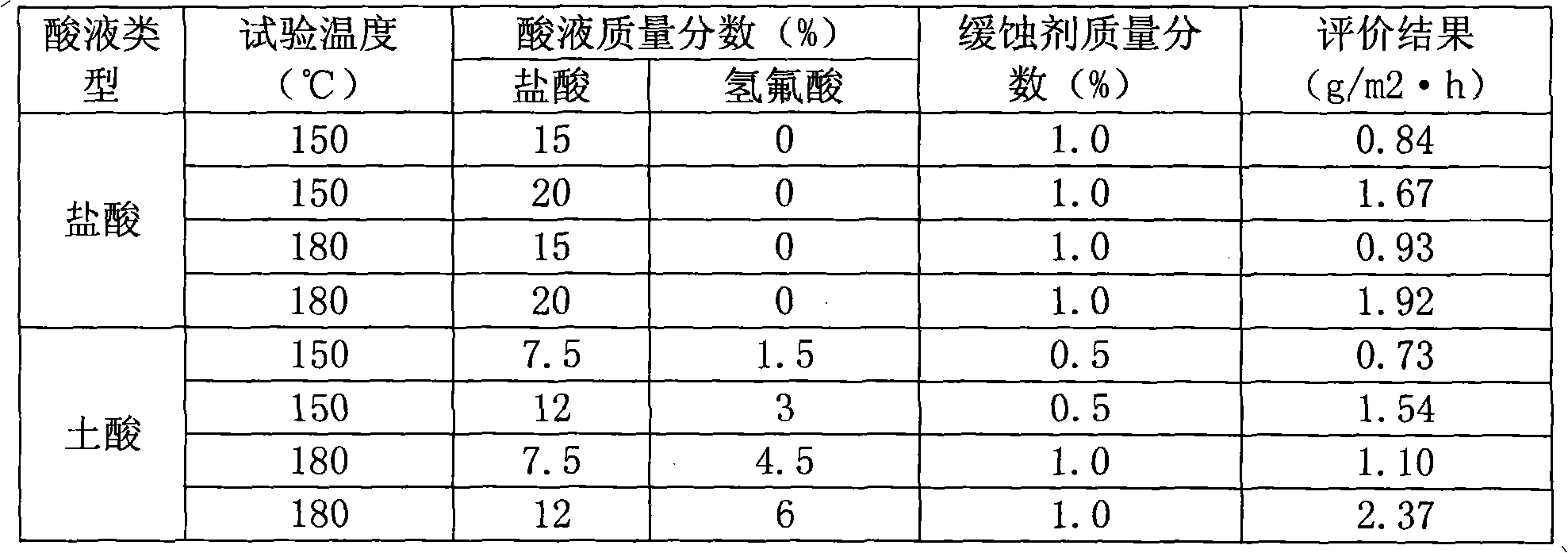
Preparation of high-temperature acidification corrosion inhibitor
The invention provides preparation of a high-temperature acidification corrosion inhibitor which is prepared from the following components in parts by weight: 50 to 60 parts of sulfathiazole solution, 10 to 15 parts of phenolic resin, 10 to 15 parts of dodecyl dimethyl benzyl ammonium chloride, 5 to 10 parts of aminopolysiloxane and 10 to 20 parts of diethylene glycol; the components for optimalpreparation comprise 60 parts of sulfathiazole, 15 parts of phenolic resin, 10 parts of dodecyl dimethyl benzyl ammonium chloride, 7 parts of aminopolysiloxane and 15 parts of diethylene glycol; and when in preparation, the components are sequentially added into a reaction kettle and stirred for 30 to 40 minutes to obtain the product, wherein the preparation of the sulfathiazole solution comprises the following steps: taking 45 to 50 parts of 2-aminothiazole and 45 to 50 parts of p-nitrobenzene sulfonyl chloride, adding into the reaction kettle, stirring, keeping the constant temperature to 40 DEG C for 2 to 3 hours, then adding 45 to 50 parts of sodium bicarbonate, and continuing to react for 4 to 5 hours to obtain the solution. The product still has good corrosion inhibiting effect and compatibility when used under the acidification condition of 150 to 180 DEG C.
Owner:XINJIANG DELAND
Preparation method of p-aminophenylacetic acid
ActiveCN103214384ALow costReduce dosageOrganic compound preparationAmino-carboxyl compound preparationPtru catalystPhenylacetic acid
The invention discloses a preparation method of a p-aminophenylacetic acid, which comprises the following steps of: adding p-nitrophenylacetic acid, ethanol and a catalyst into a pressure reaction kettle in a certain proportion, replacing air in the reaction kettle with nitrogen, feeding hydrogen into the reaction kettle, and controlling a certain pressure and temperature; after the reaction is performed for a period of time, standing the reaction kettle, cooling the obtained product to room temperature, opening the pressure reaction kettle, and extracting 60-80% solvent ethanol through evaporating; carrying out cooling crystallization, filtration and drying on the solvent ethanol so as to obtain a coarse product, and carrying out recrystallization on the coarse product by using ethanol, and carrying out active carbon decoloration on the obtained product so as to obtain white crystalline p-aminophenylacetic acid, wherein the mass ratio of the p-nitrophenylacetic acid, the ethanol to the catalyst is 1:10: (0.02-0.05), the mass concentration of the ethanol is 95%, the mass concentration of the catalyst is 3-8% Pd / attapulgite clay, the reaction pressure is 0.4-0.6 MP, the reaction time is 3-5 hours, and the reaction temperature is 30-60 DEG C. According to the invention, the catalyst is low in cost, less in application amount and convenient for recycling; the method is mild in reaction conditions, energy-saving and environment-friendly; the method is good in reaction selectivity, less in side effects, easy to realize purification, and high in yield; and through taking hydrogen as a raw material, the method is less in pollution, simple in post-processing, and low in production cost.
Owner:常熟紫金知识产权服务有限公司
Production method of malonic acid mono-p-nitrobenzyl ester as penem medicament intermediate
InactiveCN102276476ASimple processEasy to operateOrganic chemistryOrganic compound preparationMalonic acidSolvent
The invention discloses a production method of malonic acid mono-p-nitrobenzyl ester as a penem medicament intermediate. The malonic acid mono-p-nitrobenzyl ester is generated through reaction in the existence of halogenated p-nitrobenzene and malonic acid or sodium salt thereof, a solvent, a catalyst and the like. The production method disclosed by the invention has the advantages of simple process, easiness for operation and high product yield.
Owner:江苏德峰药业有限公司
P-nitrophenoxy betulin (28) formyl ester and betulin derivatives synthesized therefrom, and preparation method and application thereof
ActiveCN107892709AMild reaction conditionsImprove bioavailabilityDigestive systemSteroidsSolubilityLysosome localization
The invention discloses p-nitrophenoxy betulin (28) formyl ester and betulin derivatives synthesized therefrom, and a preparation method and an application thereof, and belongs to the technical fieldof pharmaceutical chemical. Four derivatives are synthesized with betulin as a matrix, that is to say, the tail end of a structure of the betulin is modified, a carbonacylation agent nitrophenyl chloroformate is introduced to a C-28 site, p-nitrophenoxy betulin (28) formyl ester is generated, and based on the p-nitrophenoxy betulin (28) formyl ester, four kinds of groups having biological activityand functionality are introduced. Specifically, with introduction of a structure of aminoethyl alcohol, the solubility is improved; with introduction of an N-amino morpholine group having a lysosomepositioning action and a triphenylphosphine active group having mitochondria targeted positioning, the product targetedly acts on cell organelles, the bioavailability is improved, and the higher biological activity is played; with introduction of a p-aminophenol structure, the synthesized compounds have certain antioxidant effects, and certain pharmacological activities are played by regulating the balance of redox in cells.
Owner:YANBIAN UNIV
Synthesis method of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol and salt thereof
InactiveCN103193658AReduce pollutionSuitable for industrialized mass productionOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsPhenethyl alcohol
The invention provides a synthesis method of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol and salt thereof, belongs to the technical field of medicine synthesis and solves the problems that in the prior art the cost is high, the product yield is low, the synthesis method is not applicable to large-scale industrial production, and the like. The synthesis method comprises the following steps of: under the action of an oxidant, oxidizing hydroxyl on p-nitrobenzene ethanol into an aldehyde group so as to obtain p-nitrobenzene acetaldehyde; under the action of a reducing agent, carrying out dehydration, condensation and reduction on amino on (R)-2-amino-1-phenethyl alcohol and the aldehyde group on p-nitrobenzene acetaldehyde so as to obtain (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol; and mixing a rough product of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol with an acid so as to generate precipitate or stirring till solid (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol salt is separated out. The synthesis method of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol and the salt thereof is low cost and high in product yield, and is applicable to large-scale industrial production.
Owner:SUZHOU UUGENE BIOPHARMA
Light illumination and mechanically catalytic method for degradation of p-nitrobenzenes by using nano copper-protoxide
InactiveCN1699201ASimple methodMaterials are readily availableWater/sewage treatment by irradiationEnergy based wastewater treatmentCatalytic methodWastewater
The invention discloses a light illumination and mechanically catalytic method for degradation of p-nitrobenzenes by using nano copper-protoxide comprising the steps of, (1) loading waste-water containing nitrophenyl into a reactor, (2) charging nano level cuprous oxide by the proportion of 0.50g / L-4g / L, (3) applying external force to result in the friction between friction devices and the bottom of reactors under the irradiation of light sources.
Owner:NANJING UNIV
Preparation technology of antibody drug conjugate intermediate by one-pot method
ActiveCN111620927ALow costReduce lossOrganic active ingredientsPeptide preparation methodsDrug conjugationAntiendomysial antibodies
The invention relates to a method for preparing an antibody drug conjugate intermediate by a one-pot method. A preparation technology comprises the steps of enabling MC-Val-Cit-PAB-OH and bis(4-nitrophenyl) carbonate (NPC) to be in contact for a reaction under the condition of existence of organic alkali, after the reaction is completed, supplementing and adding the organic alkali, 1-hydroxybenzotriazole and a part of drugs D in a reaction system, and performing a reaction to obtain the antibody drug conjugate intermediate. In the whole reaction system, separation and purification treatment isonly performed once, steps of concentration and washing of intermediate reaction liquid, treatment of organic waste liquid, packaging and storage of intermediates, and the like are not needed, and higher yield can be achieved.
Owner:MABPLEX INT LTD
Alkaline phosphatase detection kit
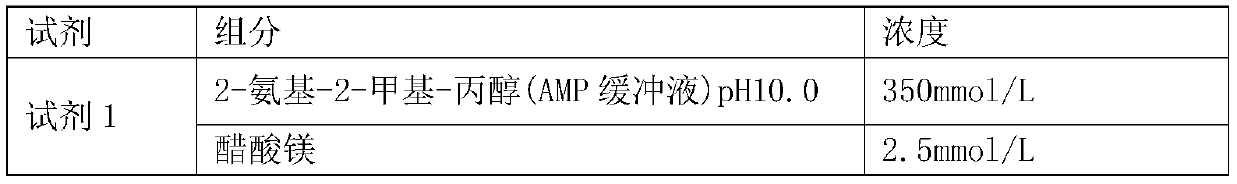
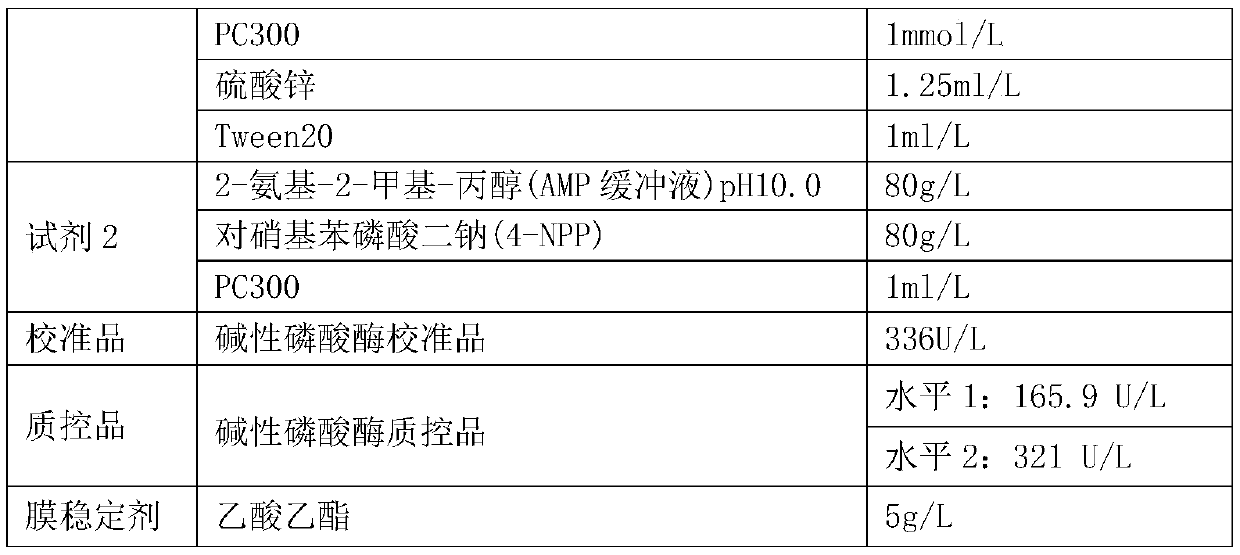
PendingCN111004833AReduce exposureGuarantee the stability of opening the lidMicrobiological testing/measurementBiological material analysisEthylic acidPhosphoric acid
The invention provides an alkaline phosphatase detection kit, and belongs to the technical field of medical examination. The kit comprises a reagent 1, a reagent 2, a calibration material and a quality control material. The reagent 1 comprises the following main components: 2-amino-2-methyl-1-propanol (AMP buffer solution with the pH value of 10.0), magnesium acetate, PC300, zinc sulfate and Tween20. The reagent 2 is prepared from the following main components: 2-amino-2-methyl-propanol (AMP buffer solution with the pH value of 10.0), disodium p-nitrophenyl phosphate (4-NPP) and PC300. By adding ethyl acetate, PMX-200 or liquid paraffin into the reagent 1 and the reagent 2, the contact between the reagents and air is effectively reduced, the reaction between the main components in the reagents and oxygen is reduced, and the uncovering stability of the reagents is guaranteed.
Owner:江苏迈源生物科技有限公司
Method for nitrifying nitrobenzene by using micro-channel continuous flow reactor
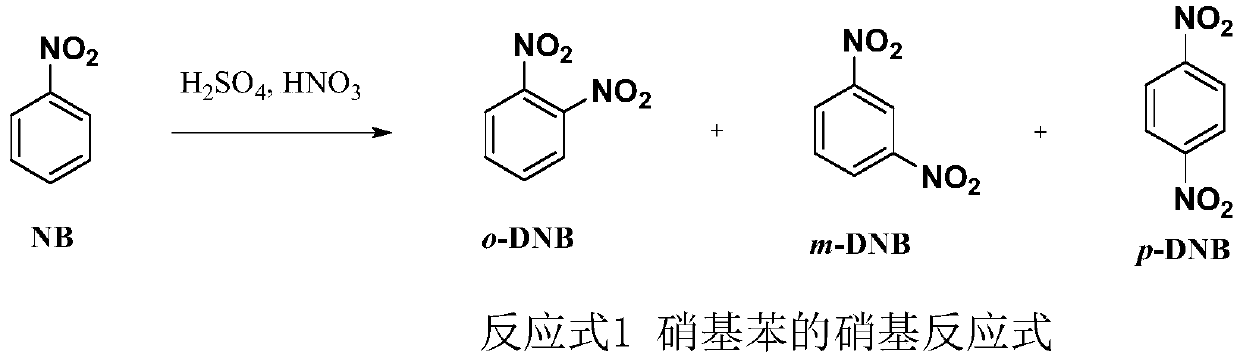
InactiveCN111393299AAchieve the purpose of reuseReduce pollutionChemical/physical/physico-chemical microreactorsNitro compound preparationNitrobenzeneNitration
The invention discloses a method for nitrifying nitrobenzene by using a micro-channel continuous flow reactor, and belongs to the technical field of preparation of chemical intermediates. The method specifically comprises the following steps: 1, preparing 65% nitric acid and 92% sulfuric acid into mixed acid according to a molar ratio of 1:3.0-10.0; 2, respectively inputting nitrobenzene and the mixed acid into a micro-channel continuous flow reactor for preheating, wherein the molar ratio of nitric acid in the nitrobenzene to nitric acid in the mixed acid is 1:1.05-1.25; 3, making the preheated nitrobenzene and the mixed acid enter a reaction module for reaction for 30-120s, and letting the reactants enter a collecting tank; and 4, performing cooling to 50-70DEG C, adding an organic solvent, stirring the substances uniformly, performing standing for layering to obtain an organic layer at the upper layer and an inorganic layer at the lower layer, and respectively conveying the organiclayer and the inorganic layer into an organic storage tank and an inorganic storage tank. According to the invention, the concentrations of nitric acid and sulfuric acid are reduced; and the added organic solvent does not reduce the concentration of sulfuric acid in the process of separating dinitrobenzene from sulfuric acid, thereby achieving the goal of sulfuric acid reutilization.
Owner:沈阳感光化工研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com