2-carbonyl-3-phenylpropionic acid p-nitrobenzoyl hydrazone di-p-methyl benzyl tin complex as well as preparation method and application thereof
A kind of technology of nitrobenzoyl hydrazone di, methylbenzyl tin, applied in 2-carbonyl-3-phenylpropionic acid-p-nitrobenzoyl hydrazone di-p-methylbenzyl tin complex and preparation and Application field, can solve problems such as undiscovered compounds, and achieve the effects of simple preparation method, low cost and high anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of 2-carbonyl-3-phenylpropionic acid p-nitrobenzoylhydrazone di-p-methylbenzyltin complex:
[0043] Add 0.400g (1.0mmol) di-p-methylbenzyl tin dichloride, 0.181g (1.0mmol) p-nitrobenzohydrazide, 0.195g (1.05mmol) phenylpyruvate into a 100mL three-necked flask protected by nitrogen Sodium and 15mL of solvent, anhydrous methanol, reacted at a temperature of 50~65°C for 8 h, cooled, filtered, and controlled solvent volatilization and crystallization at 20~35°C to obtain yellow transparent crystals, namely 2-carbonyl- 3-Phenylpropanoic acid p-nitrobenzoylhydrazone di-p-methylbenzyltin complex. Yield: 70.6%. Melting point: 86~88°C (dec).
[0044] Elemental analysis (C 66 h 66 N 6 o 12 sn 2 ): Calculated: C 57.75, H 4.85, N 6.12; Found: C 57.80, H 4.84, N 6.11.
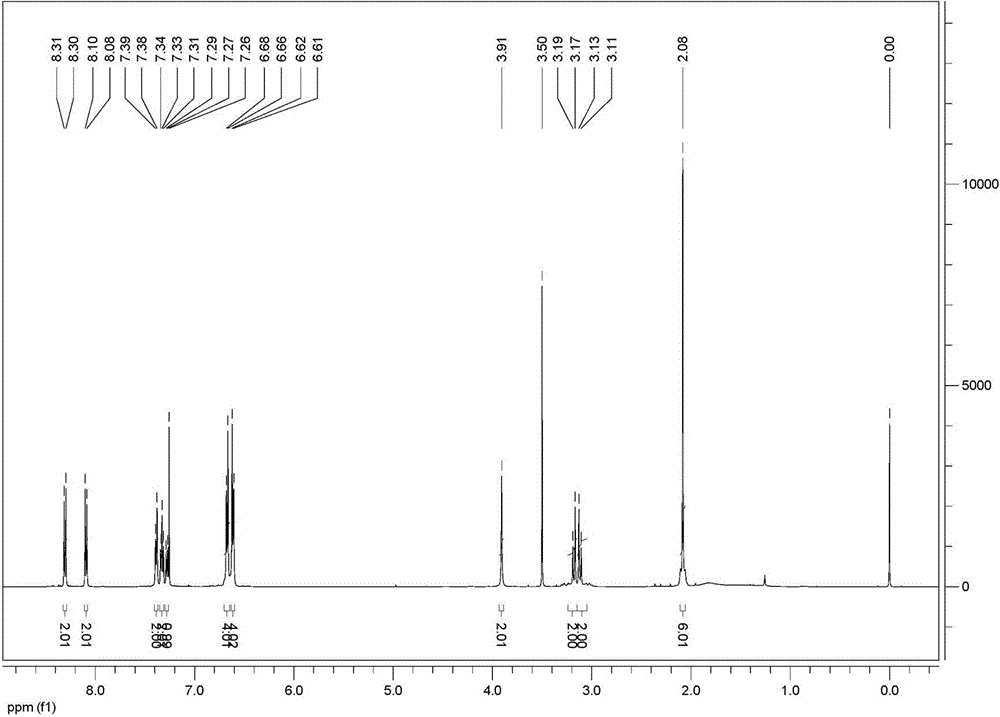
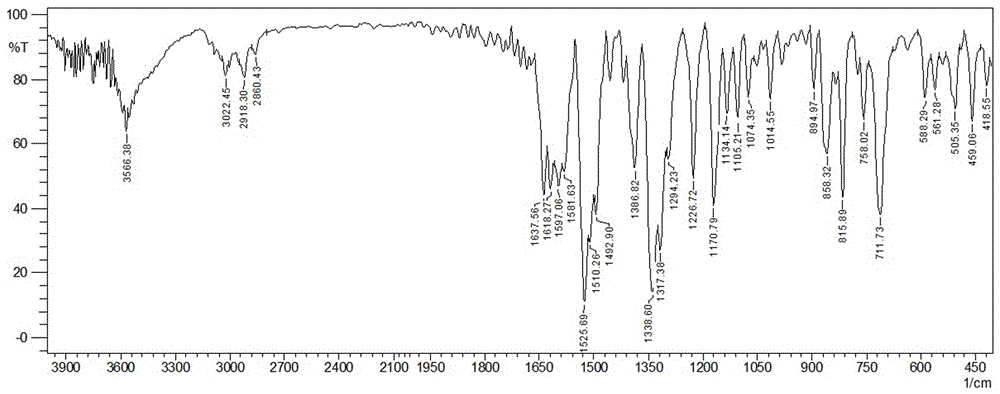
[0045] FT-IR (KBr, ν / cm -1 ): 3566, 3022, 2918, 2860, 1637, 1618, 1597, 1525, 1492, 1386, 1338, 1317, 1226, 1170, 1134, 1105, 1014, 894, 858, 5 815, 0, 51, 88, 71 459, 418.
[0046] 1 H NMR (5...
Embodiment 2
[0051] Preparation of 2-carbonyl-3-phenylpropionic acid p-nitrobenzoylhydrazone di-p-methylbenzyltin complex:
[0052] Add 0.400g (1.0mmol) di-p-methylbenzyl tin dichloride, 0.190g (1.05mmol) p-nitrobenzohydrazide, 0.214g (1.15mmol) phenylpyruvate into a 100mL three-necked flask protected by nitrogen Sodium and 35mL of solvent, anhydrous methanol, reacted for 5 hours at a temperature of 50~65°C, cooled, filtered, and controlled solvent volatilization and crystallization at 20~35°C to obtain yellow transparent crystals, namely 2-carbonyl- 3-Phenylpropanoic acid p-nitrobenzoylhydrazone di-p-methylbenzyltin complex. Yield: 73.3%. Melting point: 86~88°C (dec).
[0053] Elemental analysis (C 66 h 66 N 6 o 12 sn 2 ): Calculated: C 57.75, H 4.85, N 6.12; Found: C 57.80, H 4.84, N 6.11.
[0054] FT-IR (KBr, ν / cm -1 ): 3566, 3022, 2918, 2860, 1637, 1618, 1597, 1525, 1492, 1386, 1338, 1317, 1226, 1170, 1134, 1105, 1014, 894, 858, 5 815, 0, 51, 88, 71 459, 418.
[0055] 1 H N...
Embodiment 3
[0060] Preparation of 2-carbonyl-3-phenylpropionic acid p-nitrobenzoylhydrazone di-p-methylbenzyltin complex:
[0061] Add 2.000g (5.0mmol) di-p-methylbenzyl tin dichloride, 0.923g (5.1mmol) p-nitrobenzohydrazide, 1.023g (5.5mmol) phenylpyruvate into a 100mL three-necked flask protected by nitrogen Sodium and 25mL of solvent, anhydrous methanol, reacted at a temperature of 50~65°C for 20 h, cooled, filtered, and controlled solvent volatilization and crystallization at 20~35°C to obtain yellow transparent crystals, namely 2-carbonyl- 3-Phenylpropanoic acid p-nitrobenzoylhydrazone di-p-methylbenzyltin complex. Yield: 74.5%. Melting point: 86~88°C (dec).
[0062] Elemental analysis (C 66 h 66 N 6 o 12 sn 2 ): Calculated: C 57.75, H 4.85, N 6.12; Found: C 57.80, H 4.84, N 6.11.
[0063] FT-IR (KBr, ν / cm -1 ): 3566, 3022, 2918, 2860, 1637, 1618, 1597, 1525, 1492, 1386, 1338, 1317, 1226, 1170, 1134, 1105, 1014, 894, 858, 5 815, 0, 51, 88, 71 459, 418.
[0064] 1 H NMR (5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com