Preparation method of quinolone compounds and intermediates thereof
A compound, selected technology, applied in the direction of organic chemistry, etc., can solve problems such as complex operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
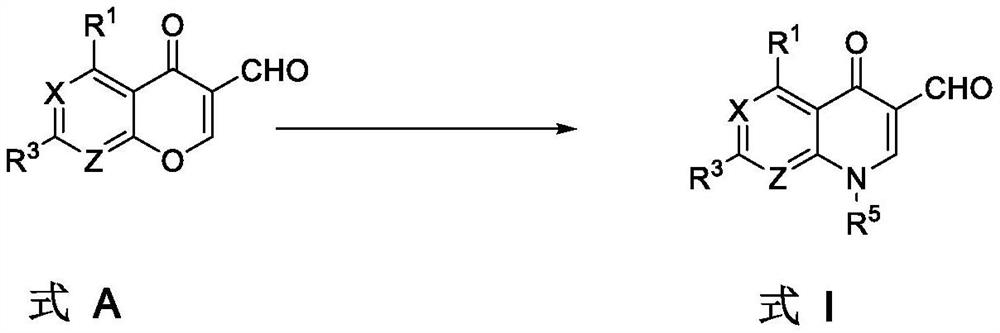
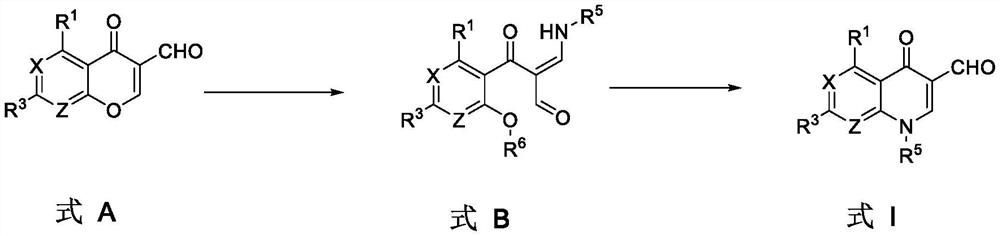
[0051] In a first aspect, the application provides a method for preparing a compound of formula I, comprising the steps of:
[0052] Combining the compound of formula A with R 5 NH 2 and R 6 Cl reacts in the presence of a base to form a compound of formula I,
[0053]
[0054] in:
[0055] X is selected from CR 2 or N;
[0056] Z is selected from CR 4 or N;
[0057] R 1 independently selected from hydrogen, alkyl, alkoxy, halogen, NO 2 , CN, optionally substituted amino, haloalkyl, aryl and heteroaryl;
[0058] R 2 and R 3 each independently selected from hydrogen, alkyl, alkoxy, halogen, amino, NO 2 , CN, haloalkyl, aryl, and heteroaryl; or R 2 with R 3 connected together to form a ring;
[0059] R 4 independently selected from hydrogen, alkyl, alkoxy, halogen, amino, NO2 , CN, haloalkyl, aryl and heteroaryl;
[0060] R 5 independently selected from alkyl optionally substituted with OH or SH, cycloalkyl optionally substituted with halogen, aryl and hetero...
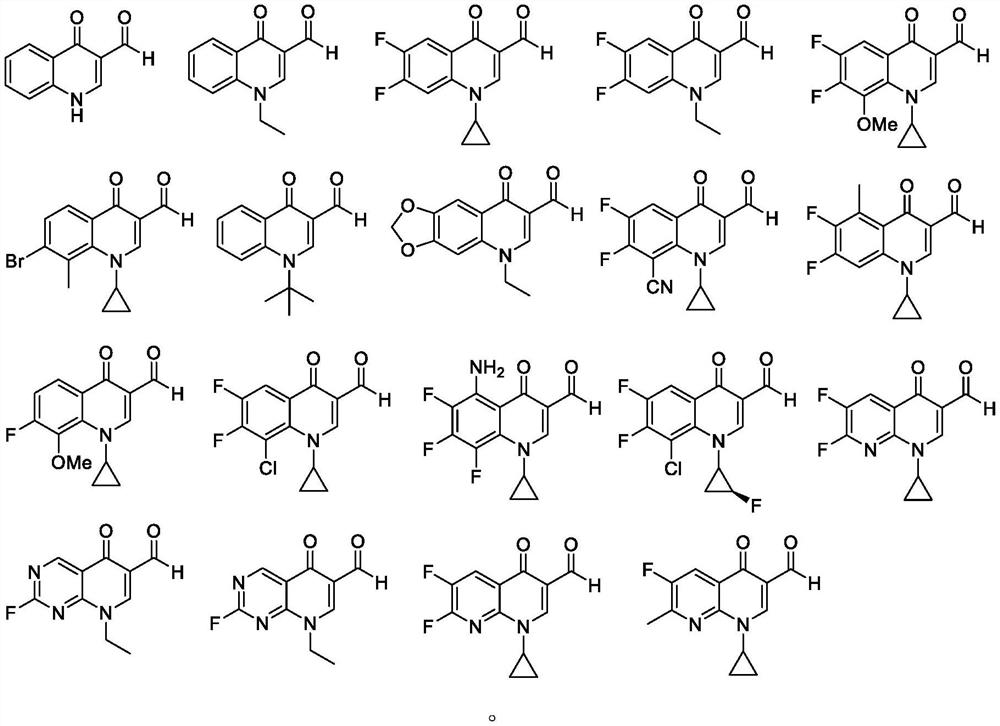
Embodiment 1
[0226] Synthesis of ciprofloxacin and grifloxacin analogs:
[0227]
[0228] The steps of concrete synthesis of ciprofloxacin are as follows:
[0229] In a 25mL round-bottomed flask, first place 6,7-difluoro-3-formylchromone (0.5mmol), cyclopropylamine (1.2mmol), p-toluenesulfonyl chloride (0.5mmol) in water (5.0mL) middle. After 5 hours at 100°C, the reaction was complete if no 6,7-difluoro-3-formylchromone and p-toluenesulfonyl chloride remained by thin layer chromatography. After the reaction solution was cooled to room temperature, ethyl acetate was added to the reaction solution, 15.0 mL each time, and extracted three times. The organic phase was dried with anhydrous sodium sulfate and separated by column chromatography to obtain the target compound 1 in a yield of 67%. 1 H NMR (400MHz, CDCl 3 )δ11.16–10.86(m,1H),9.05(d,J=1.5Hz,1H),8.07(s,1H),7.63(dt,J=8.4,1.7Hz,2H),7.23(ddd,J = 9.8, 8.4, 1.5Hz, 1H), 7.18–7.07 (m, 1H), 3.00 (ddq, J=32.7, 7.6, 3.7Hz, 1H), 2.46 (s, ...
Embodiment 2
[0234] Synthesis of Norfloxacin and Pefloxacin:
[0235]
[0236] The specific steps of synthesizing norfloxacin are as follows:
[0237] In a 25mL round-bottomed flask, first place 6,7-difluoro-3-formylchromone (0.5mmol), ethylamine (0.5mmol), p-toluenesulfonyl chloride (0.5mmol) in acetonitrile (5.0mL) Then, potassium carbonate (0.6 mmol) was weighed into the reaction system. After the addition was completed, the temperature of the reaction system was raised to 60°C, and after 5 hours, it was detected by thin layer chromatography. If no 6,7-difluoro-3-formylchromone remained, it indicated that the reaction was completed (in this step, the middle Body 6 does not need to be separated). Potassium carbonate (0.6 mmol) was added to the reaction system, and then the reaction system was heated to 120° C. and reacted for 8 hours. The reaction was monitored by thin-layer chromatography. After the reaction was completed, the reaction solution was cooled to room temperature, ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com