2-carbonyl-3-phenylpropionic-p-tert-butyl benzoyl hydrazone dibenzyl tin complex, and preparation method and application thereof
A kind of technology of tert-butylbenzoylhydrazone dibenzyltin and phenylpropionic acid, applied to 2-carbonyl-3-phenylpropionic acid p-tert-butylbenzoylhydrazone dibenzyltin complex and preparation thereof It can solve the problems of undiscovered compounds and other problems, and achieve the effects of simple preparation method, high anti-cancer activity and good anti-cancer activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of 2-carbonyl-3-phenylpropionic acid p-tert-butylbenzoylhydrazone dibenzyltin complex:
[0043] Add 0.372g (1.0mmol) dibenzyl tin dichloride, 0.192g (1.0mmol) p-tert-butylbenzohydrazide, 0.195g (1.05mmol) sodium phenylpyruvate and 15mL of anhydrous methanol as a solvent, react for 8 hours at a temperature of 50~65°C, cool, filter, and control the solvent volatilization and crystallization at a temperature of 20~35°C to obtain a yellow transparent crystal, which is 2-carbonyl-3- Phenylpropionic acid dibenzyltin complex with p-tert-butylbenzoylhydrazone. Yield: 80.6%. Melting point: 115~117°C (dec).
[0044] Elemental analysis (C 70 h 76 N 4 o 8 sn 2 ): Calculated: C 62.80, H 5.72, N 4.18; Found: C 62.86, H 5.70, N 4.17.
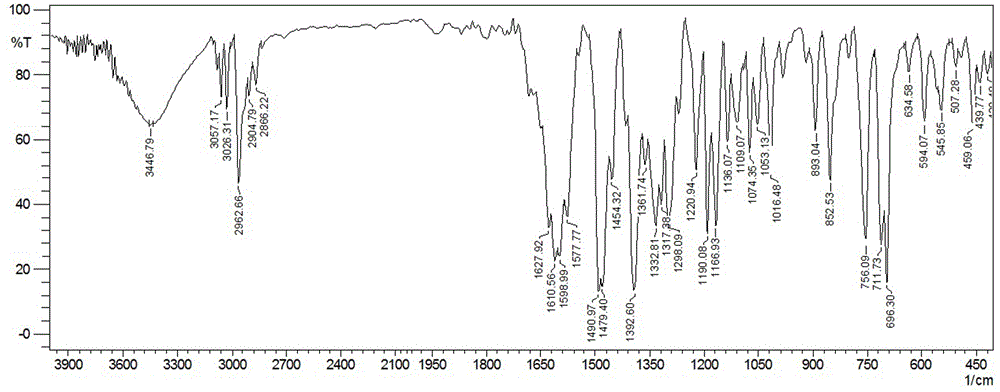
[0045] FT-IR (KBr, ν / cm -1 ): 3446, 3057, 3026, 2962, 1610, 1598, 1490, 1454, 1392, 1332, 1298, 1190, 1166, 1016, 893, 852, 756, 711, 696, 634, 594, 5445, 35, 408.
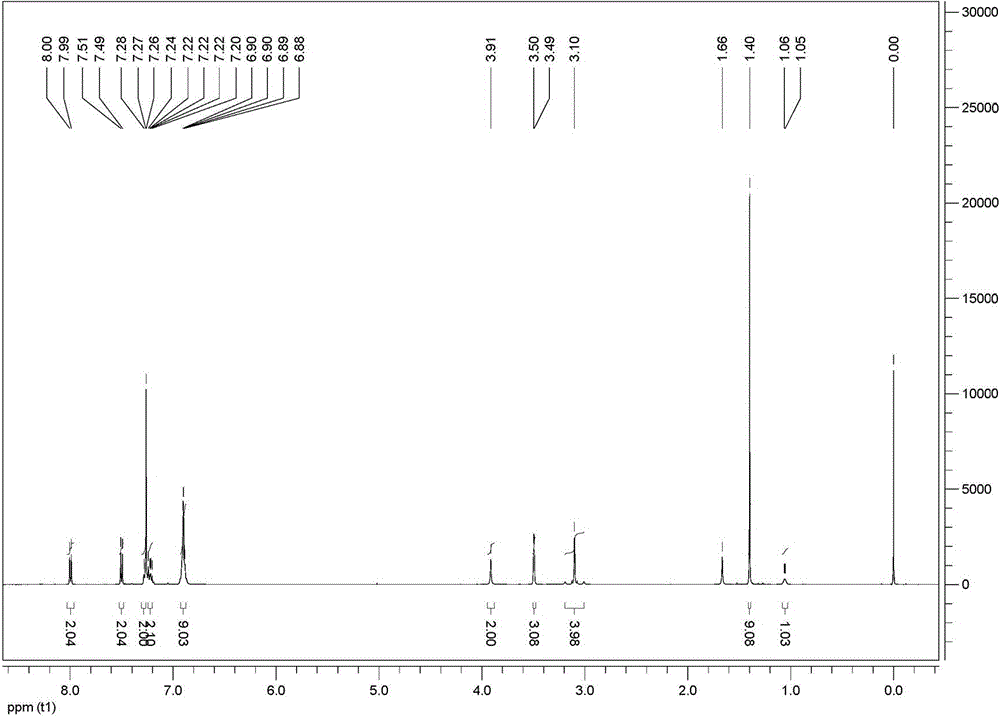
[0046] 1 H NMR (500 MHz, CDCl 3 , δ / ppm): 7.99 (d, J =8.5 Hz...
Embodiment 2
[0051] Preparation of 2-carbonyl-3-phenylpropionic acid p-tert-butylbenzoylhydrazone dibenzyltin complex:
[0052] Add 0.372g (1.0mmol) dibenzyltin dichloride, 0.202g (1.05mmol) p-tert-butylbenzohydrazide, 0.214g (1.15mmol) sodium phenylpyruvate and 35mL of anhydrous methanol as a solvent, react for 5 hours at a temperature of 50~65°C, cool, filter, and control the solvent volatilization and crystallization at a temperature of 20~35°C to obtain a yellow transparent crystal, which is 2-carbonyl-3- Phenylpropionic acid dibenzyltin complex with p-tert-butylbenzoylhydrazone. Yield: 82.5%. Melting point: 115~117°C (dec).
[0053] Elemental analysis (C 70 h 76 N 4 o 8 sn 2 ): Calculated: C 62.80, H 5.72, N 4.18; Found: C 62.86, H 5.70, N 4.17.
[0054] FT-IR (KBr, ν / cm -1 ): 3446, 3057, 3026, 2962, 1610, 1598, 1490, 1454, 1392, 1332, 1298, 1190, 1166, 1016, 893, 852, 756, 711, 696, 634, 594, 5445, 35, 408.
[0055] 1 H NMR (500 MHz, CDCl 3 , δ / ppm): 7.99 (d, J =8.5 Hz...
Embodiment 3
[0060] Preparation of 2-carbonyl-3-phenylpropionic acid p-tert-butylbenzoylhydrazone dibenzyltin complex:
[0061] Add 1.860g (5.0mmol) dibenzyltin dichloride, 0.979g (5.1mmol) p-tert-butylbenzohydrazide, 1.023g (5.5mmol) sodium phenylpyruvate and 25mL solvent anhydrous methanol, react at a temperature of 50~65°C for 20 h, cool, filter, and control the solvent volatilization and crystallization at a temperature of 20~35°C to obtain a yellow transparent crystal, which is 2-carbonyl-3- Phenylpropionic acid dibenzyltin complex with p-tert-butylbenzoylhydrazone. Yield: 82.8%. Melting point: 115~117°C (dec).
[0062] Elemental analysis (C 70 h 76 N 4 o 8 sn 2 ): Calculated: C 62.80, H 5.72, N 4.18; Found: C 62.86, H 5.70, N 4.17.
[0063]FT-IR (KBr, ν / cm -1 ): 3446, 3057, 3026, 2962, 1610, 1598, 1490, 1454, 1392, 1332, 1298, 1190, 1166, 1016, 893, 852, 756, 711, 696, 634, 594, 5445, 35, 408.
[0064] 1 H NMR (500 MHz, CDCl 3 , δ / ppm): 7.99 (d, J =8.5 Hz, 2H), 7.50 (d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com