Nitrogen-doped porous carbon supported metal M@CN composite catalytic material, preparation method and application
A nitrogen-doped porous carbon, catalytic material technology, applied in the preparation of amino compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of a large amount of waste, harsh reaction conditions, etc. Mild, high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Co(NO 3 ) 2 ·6H 2 O, H 2 Dissolve BDC and BPY in a mixed solution of 10mL methanol and 20mL DMF, and fully dissolve to obtain a clear solution. The concentrations of the above three substances in the solution are 0.04mol / L, 0.05mol / L, and 0.02mol / L, respectively;
[0030] Seal the above solution in a hydrothermal reaction kettle and put it in an oven at 130°C for 72 hours; after the reaction, the hydrothermal reaction kettle was slowly lowered to room temperature in the oven, opened and filtered to obtain the product Co 2 (BDC) 2 (BPY). Wash several times with DMF to remove unreacted substances attached to the surface.
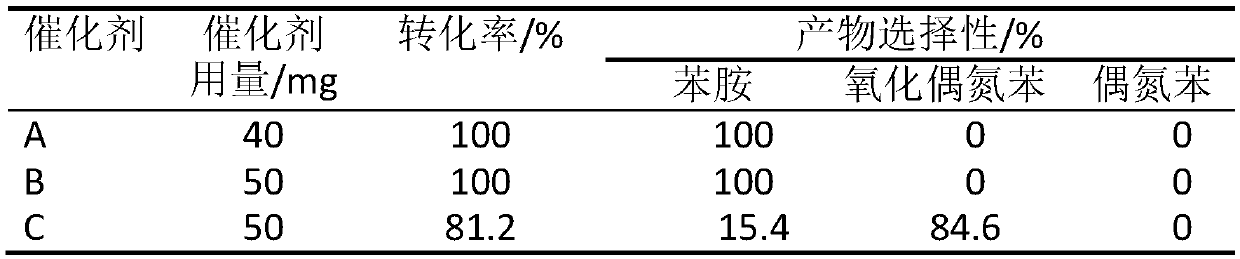
[0031] In an inert atmosphere, the above product was heated to 600 °C at a rate of 4 °C / min in a tube furnace, kept at a constant temperature for 4 hours, and then lowered to room temperature to prepare Co@CN composite catalytic material A.
Embodiment 2
[0033] Cu(NO 3 ) 2 ·3H 2 O, H 2 Dissolve BDC and BPY in a mixed solution of 20mL methanol and 10mL DMF, and fully dissolve to obtain a clear solution. The concentrations of the above three substances in the solution are 0.05mol / L, 0.05mol / L, and 0.03mol / L, respectively;
[0034] Seal the above solution in a hydrothermal reaction kettle and put it in an oven at 140°C to react for 48 hours; after the reaction, the hydrothermal reaction kettle was slowly lowered to room temperature in the oven, opened and filtered to obtain the product Cu 2 (BDC) 2 (BPY). Wash several times with DMF to remove unreacted substances attached to the surface.
[0035] In an inert atmosphere, the above product was heated to 700 °C at 2 °C / min in a tube furnace, kept at a constant temperature for 3 hours, and then lowered to room temperature to prepare Cu@CN composite catalytic material B.
Embodiment 3
[0037] Ni(NO 3 ) 2 ·6H 2 O, H 2 Dissolve BDC and BPY in a mixed solution of 15mL methanol and 15mL DMF, and fully dissolve to obtain a clear solution. The concentrations of the above three substances in the solution are 0.10mol / L, 0.10mol / L, and 0.05mol / L, respectively;
[0038] Seal the above solution in a hydrothermal reaction kettle and put it in a 120°C oven for 24 hours of reaction; after the reaction, the hydrothermal reaction kettle was slowly lowered to room temperature in the oven, opened and filtered to obtain the product Ni 2 (BDC) 2 (BPY). Wash several times with DMF to remove unreacted substances attached to the surface.
[0039] In an inert atmosphere, the above product was heated to 400 °C at a rate of 3 °C / min in a tube furnace, kept at a constant temperature for 2 hours, and then lowered to room temperature to prepare Ni@CN composite catalytic material C.
[0040] The above nitrogen-doped porous carbon-supported metal-supported M@CN composite catalytic m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com