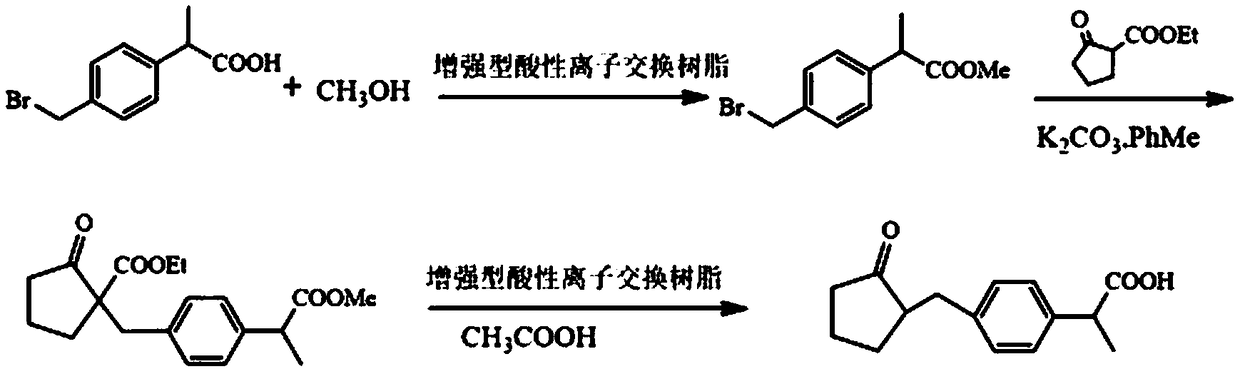
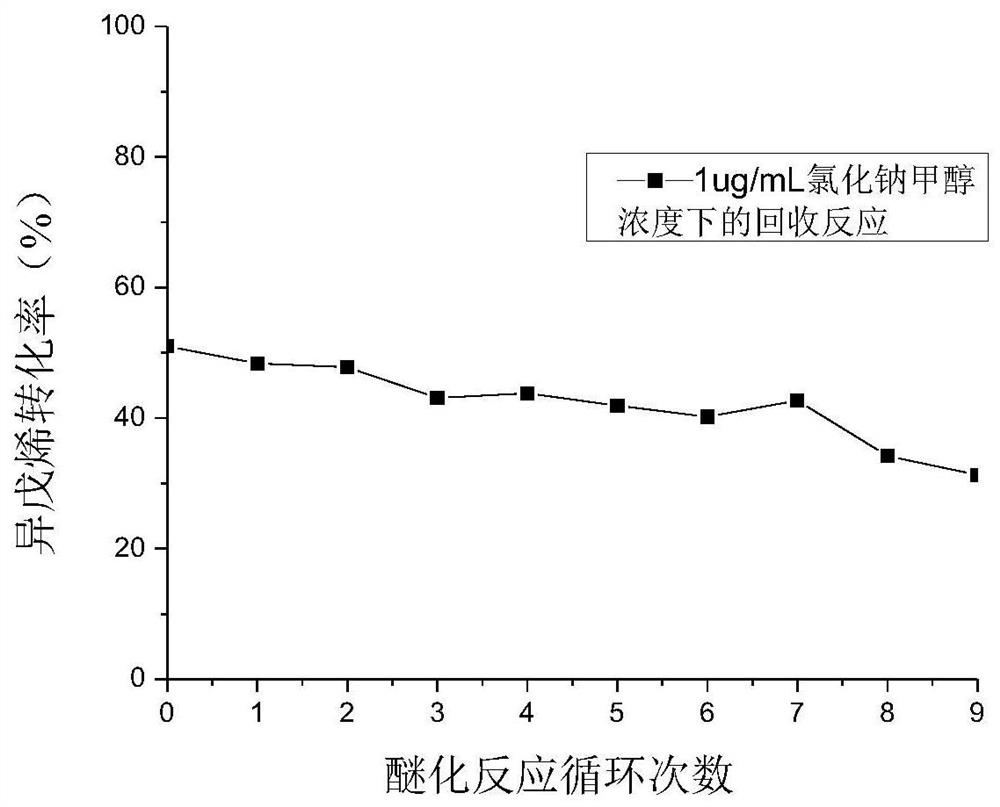
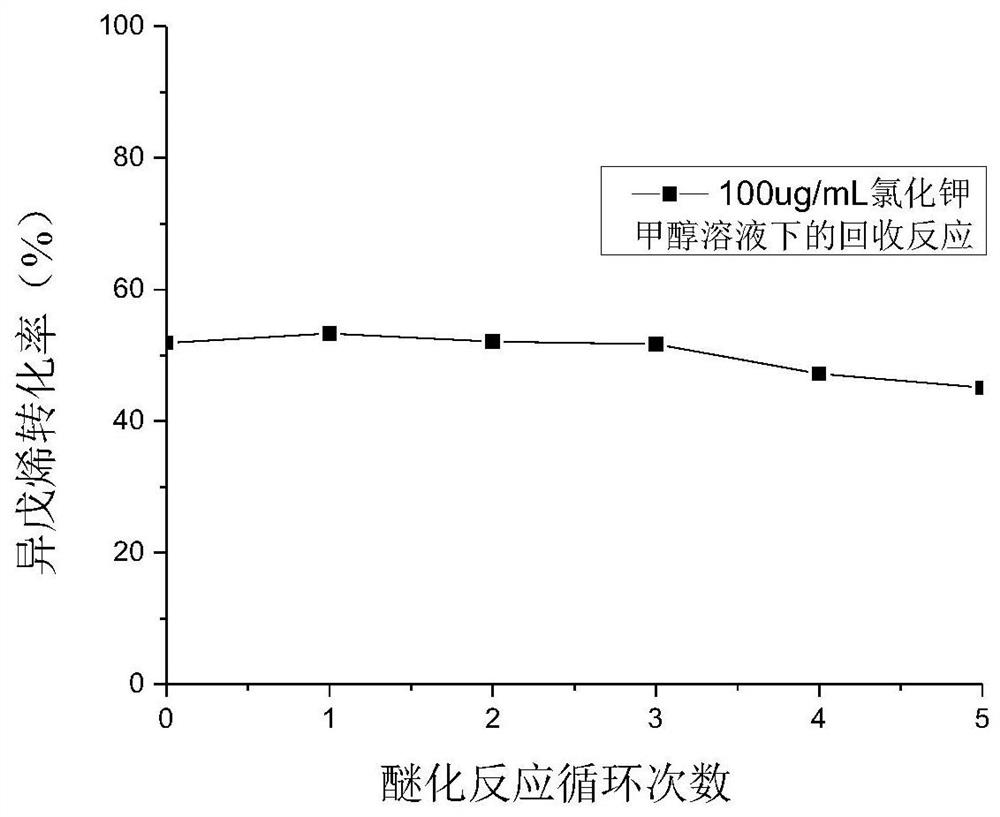
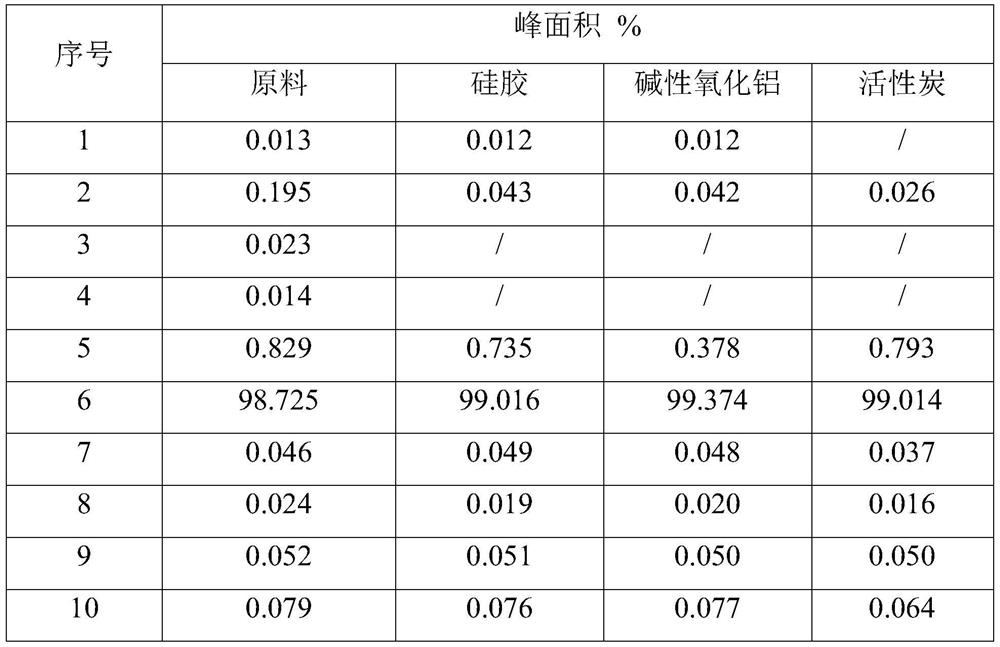
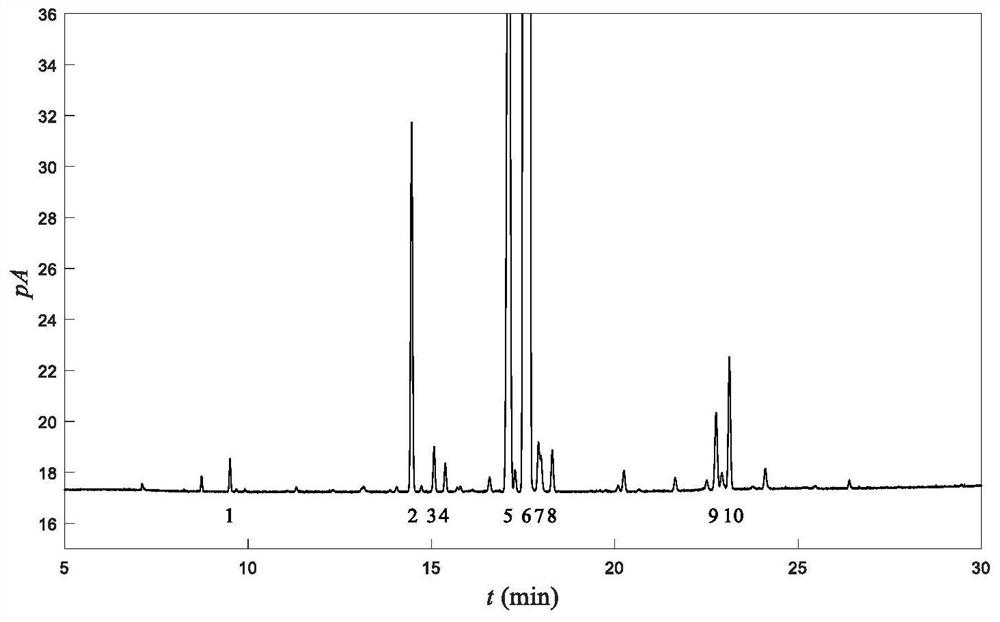
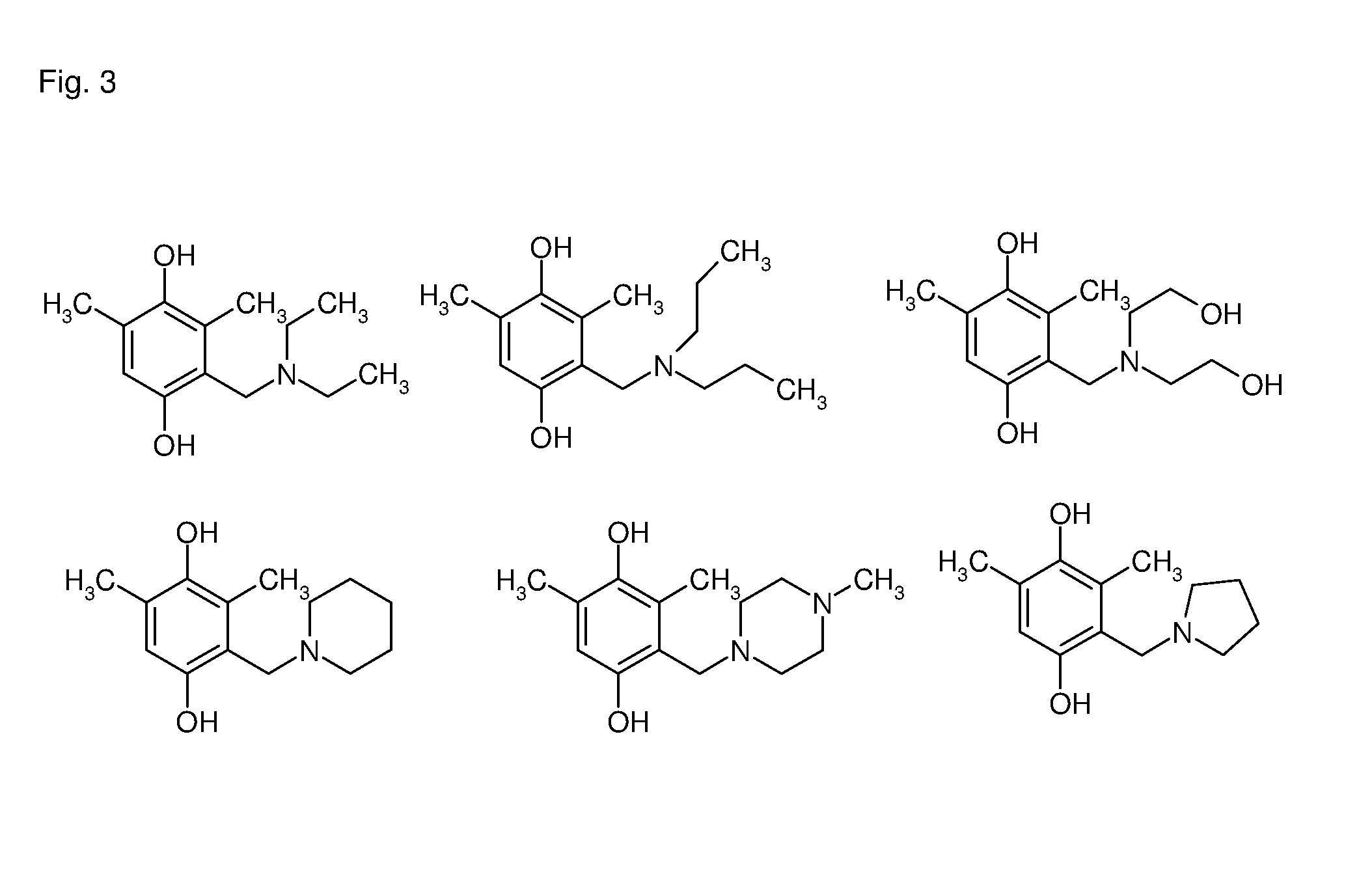
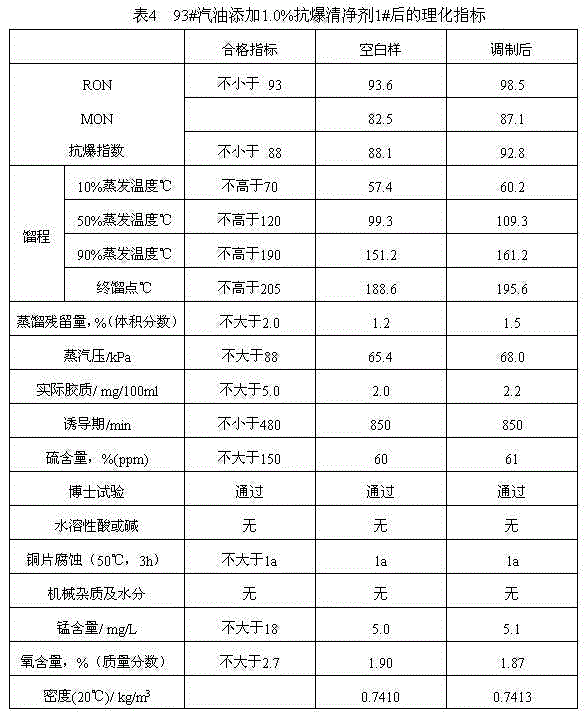
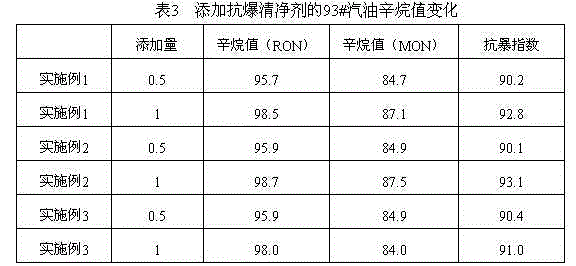
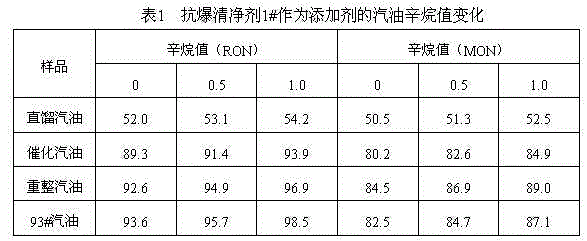
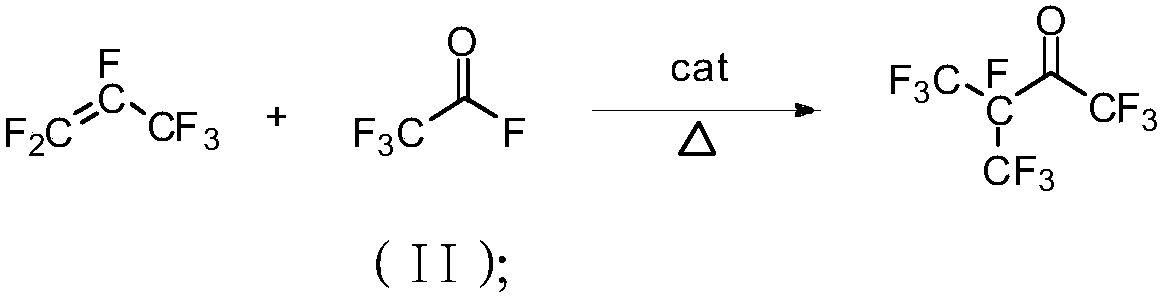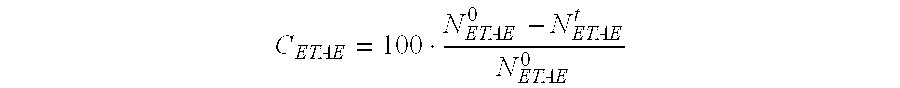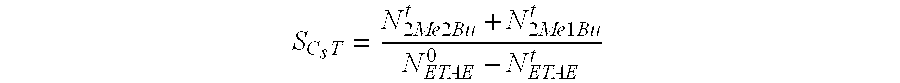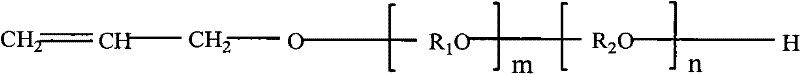Patents
Literature
32 results about "Amyl ether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for obtaining high pure dicarboxylic acid
InactiveCN102911036AHigh purityReduce consumptionCarboxylic compound separation/purificationPropyl etherCarboxylic acid
The invention relates to a method for obtaining high pure dicarboxylic acid. The method comprises the steps of I. heating a termination fermentation broth for inactivation; II. acidizing to make dicarboxylic acid crystallized and precipitated, and filtering to obtain a filter cake of dicarboxylic acid; III. mixing the filter cake of dicarboxylic acid and an ether solvent to make the dicarboxylic acid dissolved, separating an organic phase and a water phase, wherein the ether solvent is ether, n-propyl ether, iso-propyl ether, butyl ether, amyl ether or hexyl ether; IV. adding organic an adsorbent to the organic phase obtained from the step III, and filtering to remove solid materials; and V. cooling the organic phase obtained from the step IV until dicarboxylic acid is crystallized and precipitated, filtering to obtain a filter cake of crystallized dicarboxylic acid, and drying the filter cake of crystallized dicarboxylic acid to obtain a dicarboxylic acid product with a purity, by weight, higher than 98.5%. The method overcomes the shortages of complex operation, high production cost, low product purity, high colourity, high equipment investment and the like in a conventional refining method.
Owner:CHINA PETROLEUM & CHEM CORP +1
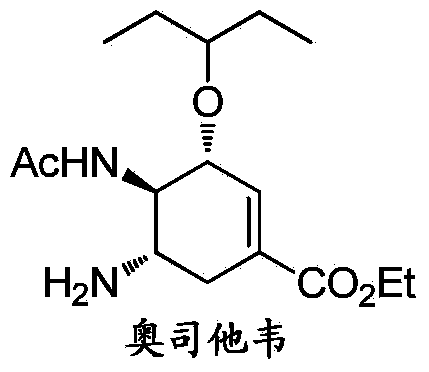
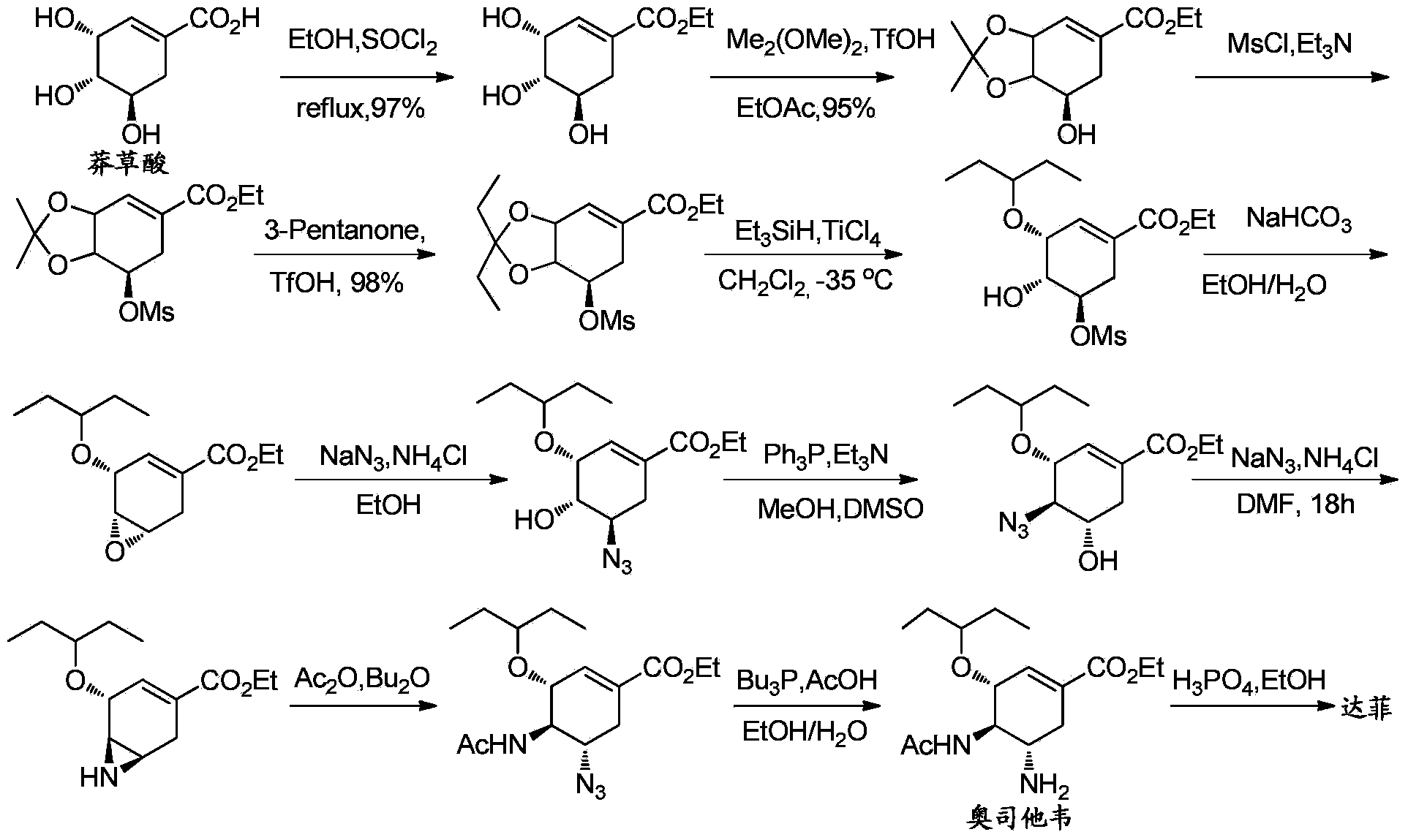
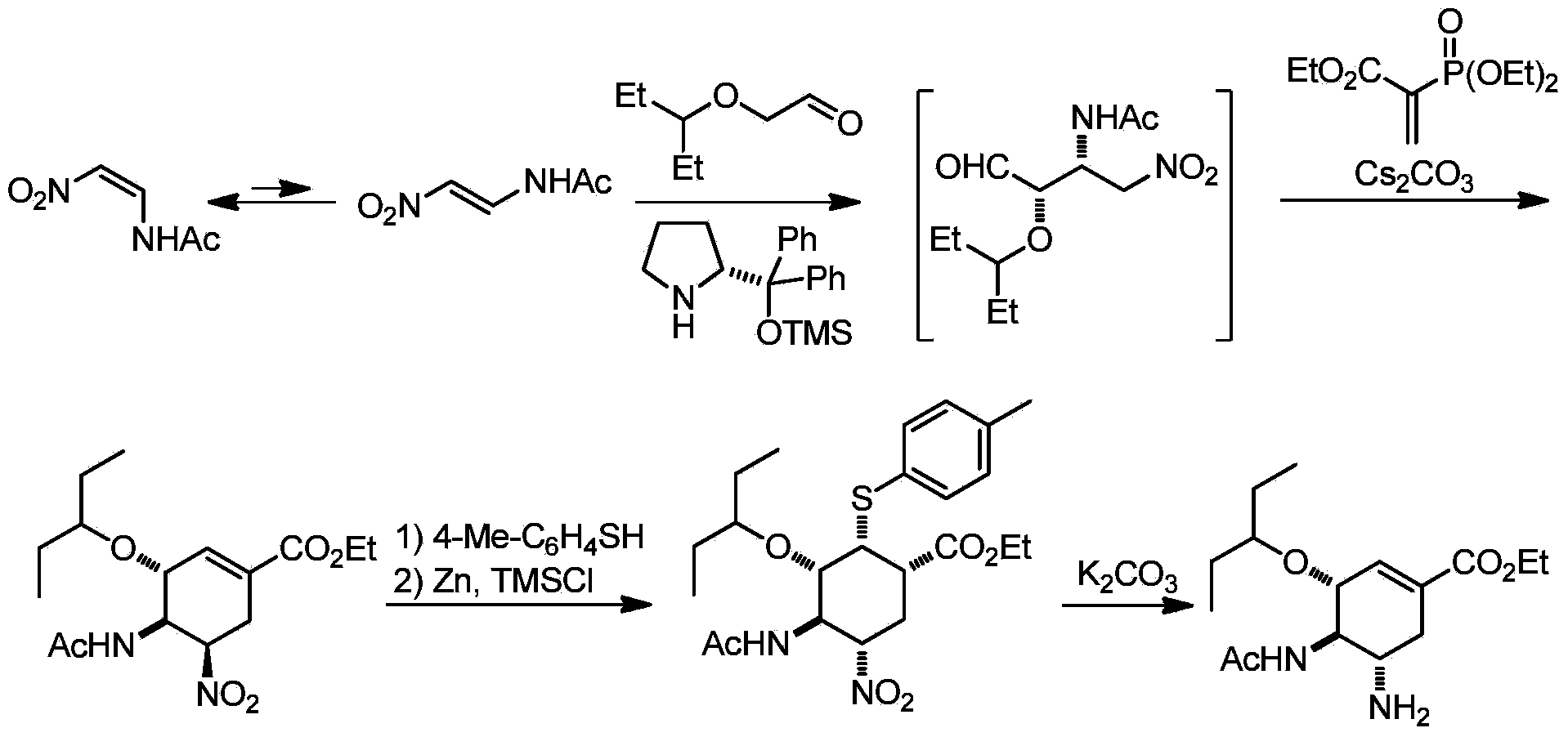
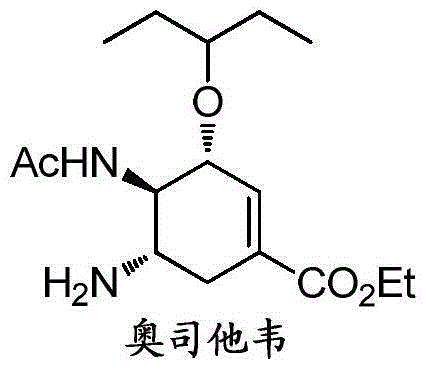
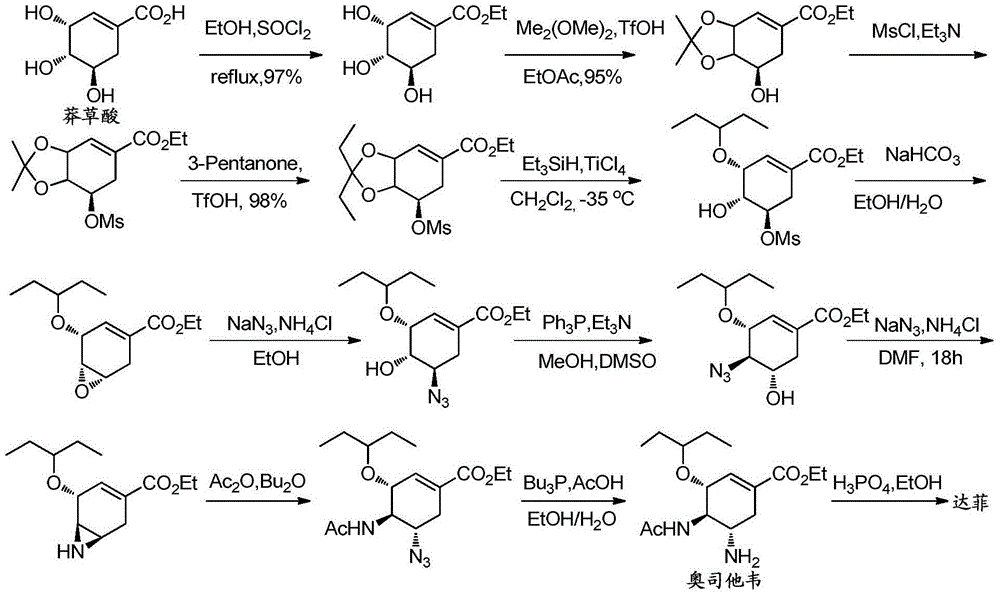
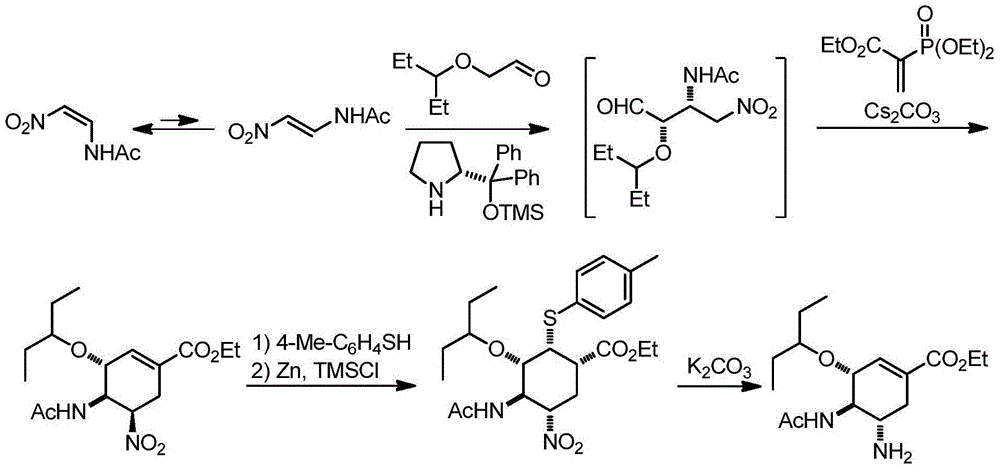
Synthesis method of oseltamivir
InactiveCN103833570AHigh yieldEasy to operateOrganic compound preparationCarboxylic acid amides preparationBird fluSynthesis methods
The invention discloses a synthesis method of oseltamivir. The synthesis method of oseltamivir comprises the following steps: starting from a compound 1,3-butadiene-3-amyl ether and compound 3-nitro-ethyl acrylate, carrying out Diels-Alder reaction, then reacting at room temperature in acetonitrile in the presence of a copper catalyst and PhI-NNs to prepare an aziridine compound in a one-pot method, wherein the mole ratio of the 1,3-butadiene-3-amyl ether to 3-nitro-ethyl acrylate to the copper catalyst is 1.1: 1: 0.025-0.1; and finally synthesizing the oseltamivir for preventing bird flu through the aziridine ring opening, nitryl and p-nitrobenzene sulfonyl removal, acetylation and hydrogenation. The method comprises short steps, the used reagent is cheap and easily available, the operation is simple, the total yield is up to 40%, and the method is a simple and efficient synthesis method of oseltamivir.
Owner:ZHEJIANG NORMAL UNIVERSITY
Method for preparing high-quality mercaptoacetic acid from tail solution from O-alkyl-N-alkyl thinocarbamate production
ActiveCN105254547AHigh recovery rateHigh purityOrganic chemistryOrganic compound preparationGlutaric acidSuspended organic matter
The invention discloses a method for preparing high-quality mercaptoacetic acid from a tail solution from O-alkyl-N-alkyl thinocarbamate production. The method comprises the following steps: (1) performing acidizing treatment on the tail solution by using an inorganic acid, and controlling the pH value to be 0.5-1.5; (2) leaving to stand for 6-48 hours, separating suspended organic matters, extracting by using an organic solvent, and extracting by using an extraction agent, thereby obtaining an extraction liquid, wherein the volume ratio of the total amount of the organic solvent to an acidifying liquid is (2.5:0.01)-(2.5:0.1), the organic solvent consists of 40-60wt% of glutaric acid diethyl ester and 40-060wt% of ethyl malonic acid diethyl ester, the volume ratio of the total amount of the extracting agent to a new acidifying liquid is (2:0.1)-(2:2), and the extracting agent is prepared by mixing n-amyl ether, isoamyl ether and sec-butyl methyl ether in a mass ratio of (1-3):(1-3):(1-3); (3) removing the solvent and water from the extraction liquid, thereby obtaining crude acid; (4) performing flash evaporation on the crude acid. The method is high in recycling rate, high in product purity, good in quality and simple in process.
Owner:QINGDAO LNT CHEM
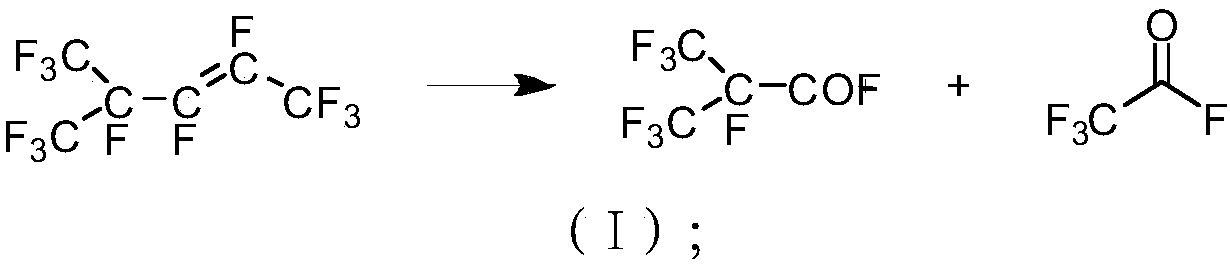
Method for cracking hexafluoropropylene dipolymer to prepare perfluoro amyl ether and perfluoro amyl ether
InactiveCN109503364AProduct highThe product perfluoroacetyl fluoride is highOrganic compound preparationCarbonyl compound preparation by condensationChemical industryHexafluoropropylene
The invention belongs to the field of fluorine chemical industry and specifically relates to a method for cracking hexafluoropropylene dipolymer to prepare perfluoro amyl ether and perfluoro amyl ether. The preparation method comprises the steps: 1) perfluoro pentacarbonyl ketone preparation: 1.1) sending the hexafluoropropylene dipolymer into an oxidizing furnace to perform cracking reaction withoxygen under the first catalyst condition; separating a cracked product to obtain perfluoro acetylfluoride; 1.2) preparing the perfluoro pentacarbonyl ketone under the second catalyst action with hexafluoropropylene and the perfluoro acetylfluoride prepared in the step 1.1) as raw materials; 2) selectively adding tertiary amine or a phase transfer catalyst into an inert dipole non-proton organiccompound solution to react under the alkali metal fluoride action with the pentacarbonyl ketone obtained in the step 1) and an alkylation reagent as the raw materials, so as to prepare the perfluoro amyl ether. According to the method disclosed by the invention, hexafluoropropylene tripolymer is utilized as a raw material to generate the perfluoro pentacarbonyl ketone, and then the perfluoro pentacarbonyl ketone reacts with the alkylation reagent to generate the perfluoro amyl ether; thus, the conversion efficiency is high, conditions are moderate, and the whole technological process is simpleto operate.
Owner:TIANJIN CHANGLU CHEM NEW MATERIAL CO LTD
Process for cracking tert-alkyl ethers that use a mesostructured hybrid organic-inorganic material
InactiveUS20090326300A1Facilitated DiffusionImprove performanceMolecular sieve catalystsOrganic-compounds/hydrides/coordination-complexes catalystsTert-Amyl methyl etherMaximum diameter
A process for cracking tert-alkyl ether(s) selected from among tert-amyl methyl ether (TAME) and ethyl tert-amyl ether (ETAE) for the production of tertiary olefins comprising bringing said tert-alkyl ether(s) into contact with at least one catalyst that is formed by at least one mesostructured hybrid organic-inorganic material that consists of at least two spherical elementary particles, whereby each of said spherical particles consists of a mesostructured matrix with a silicon oxide base to which are linked organic groups with acid terminal reactive functions, said groups representing less than 20 mol % of said matrix that is present in each of said spherical elementary particles, which have a maximum diameter of between 50 nm and 200 μm.
Owner:INST FR DU PETROLE
Catalyst for preparing polyalkyl glycol allyl amyl ether and preparation method thereof
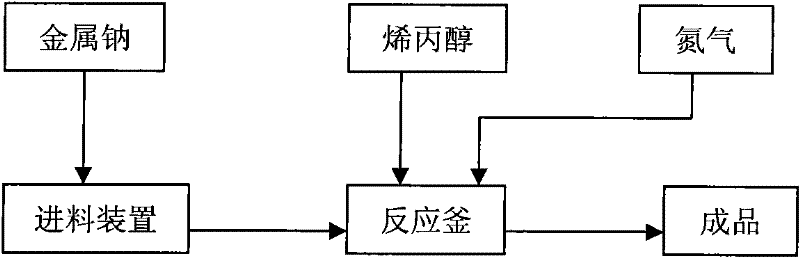
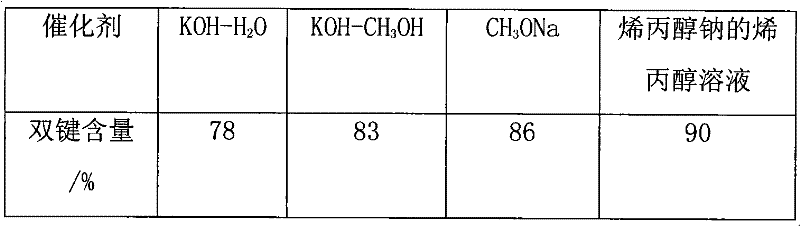
The invention discloses a catalyst for preparing polyalkyl glycol allyl amyl ether, which is an allyl alcohol solution of sodium allylate of which the mass percent concentration in the solution is 7%-37%. The preparation method comprises the following steps of: (1) putting allyl alcohol into a reaction kettle, chipping sodium metal and putting into a charging device; (2) performing nitrogen displacement, keeping an air release valve in an open state and introducing nitrogen; and (3) introducing cooling water into a condenser, adding the sodium metal in batches, controlling the temperature of the reaction system not to exceed 130 DEG C, closing a charging valve after adding the sodium metal, continuing reaction until the reaction liquid is free of bubbles, closing the air release valve, stopping introducing nitrogen, and discharging. In the process of preparing the polyalkyl glycol allyl amyl ether by the catalyst, byproducts can not be generated, thus effectively improving the purity of the polyalkyl glycol allyl amyl ether.
Owner:SHANXI UNIV
Method for manufacturing environment-friendly alcohol gasoline
InactiveCN101792683AGood stability without delaminationNo corrosionLiquid carbonaceous fuelsPhenolMethyl oleate
The invention discloses a method for manufacturing alcohol gasoline, comprising the following steps: mixing amyl alcohol and amyl ether in a certain proportion in 95% of industrial alcohol to obtain denatured alcohol for later use; proportionally selecting fatty alcohol-polyoxyethylene ether, dimethyl carbonate, ethyl oleate and para-tertiary butyl phenol; adding the selected substances into a vessel in sequence to be stirred for 20-30min to prepare a alcohol gasoline modifier for later use; and adding 70% of gasoline into 26% of denatured alcohol to be fully stirred until the mixture is uniform, then adding 4% of the gasoline modifier and fully mixing the mixture, thus obtaining the finished alcohol gasoline. The product is unstratified, has good stability, no corrosion and swelling, strong explosive power, easily obtained raw materials and low manufacturing cost, is not emulsified and is convenient to use.
Owner:唐山市隆泰科技有限公司
A kind of method of synthesizing ethyl tertiary amyl ether
ActiveCN103787841BOrganic-compounds/hydrides/coordination-complexes catalystsEther preparation by compound additionPtru catalystReaction temperature
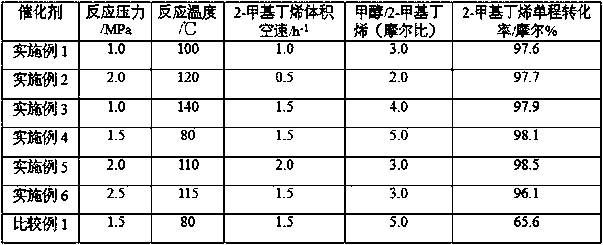
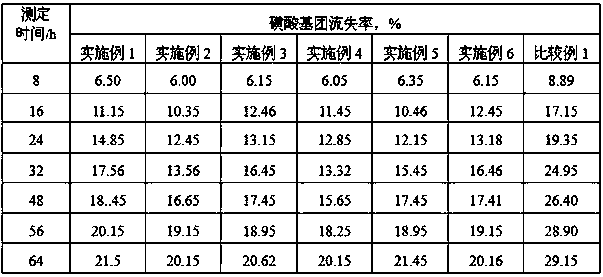
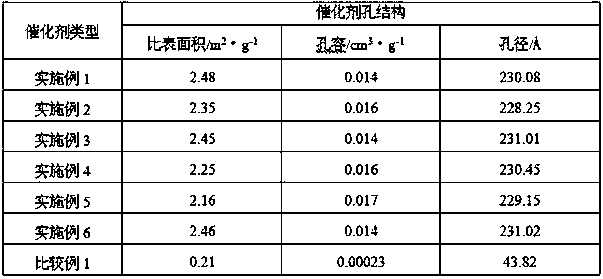
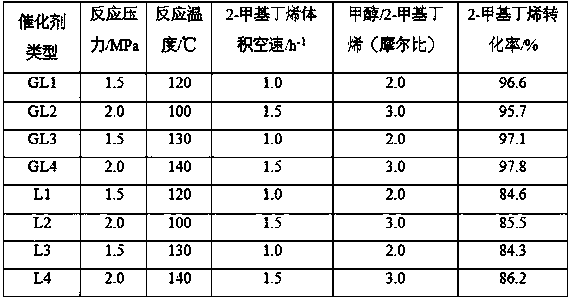
The invention discloses a synthetic method of tert-amyl ethyl ether (TAEE). The method uses 2-methyl butene and ethyl alcohol as raw materials, and aluminum based composite metal oxide loaded with cesium acid phosphotungstate as a catalyst, and carries out synthesis of TAEE under the following conditions: reaction temperature of 100-180 DEG C, reaction pressure of 2.0-6.0MPa, liquid hourly volume space velocity of 2-methyl butene of 1.0-5.0 / h, and molar ratio of ethanol and 2-methyl-butane of 1.0:1-8.0:1. The method uses a fixed bed process for continuous synthesis of TAEE, and has the advantages of high conversion rate of raw materials and long running period.
Owner:CHINA PETROLEUM & CHEM CORP +1
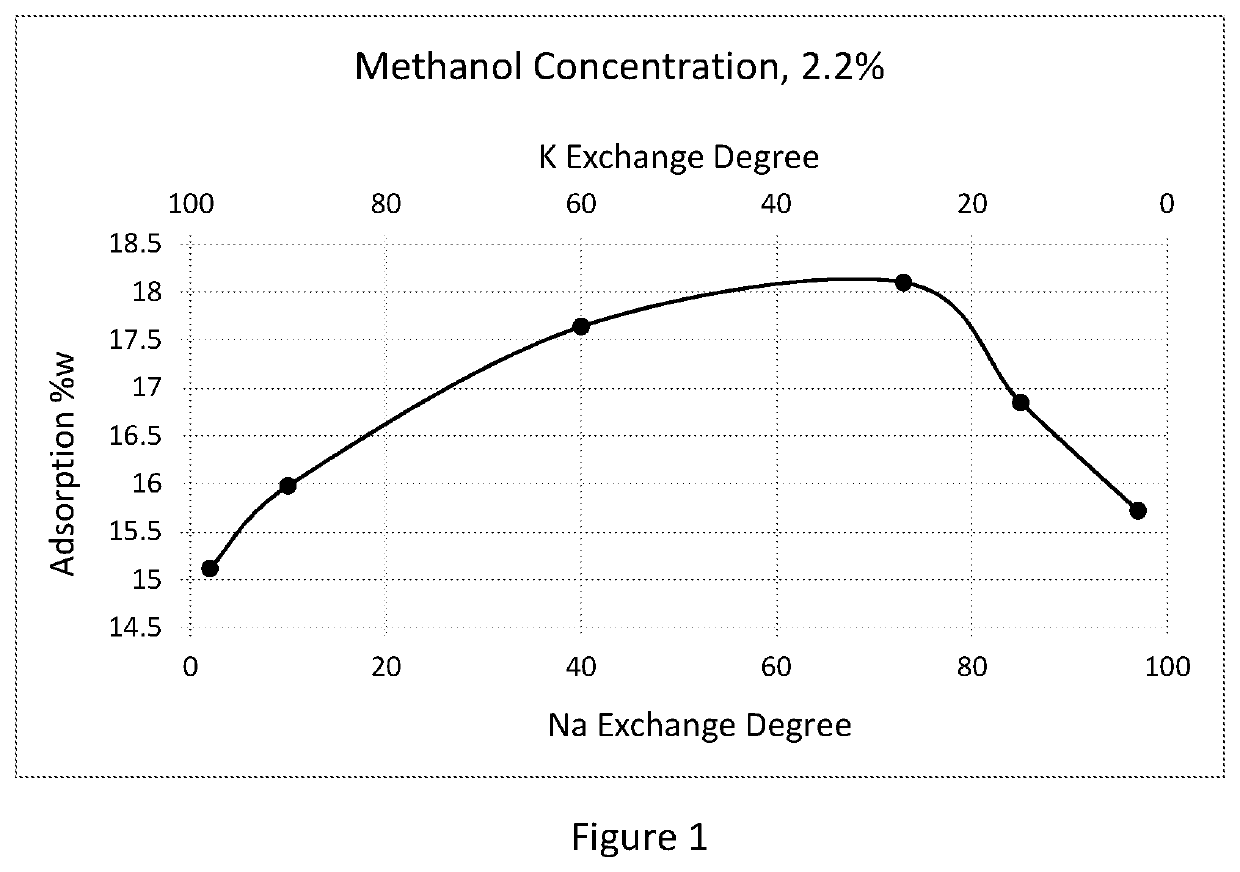
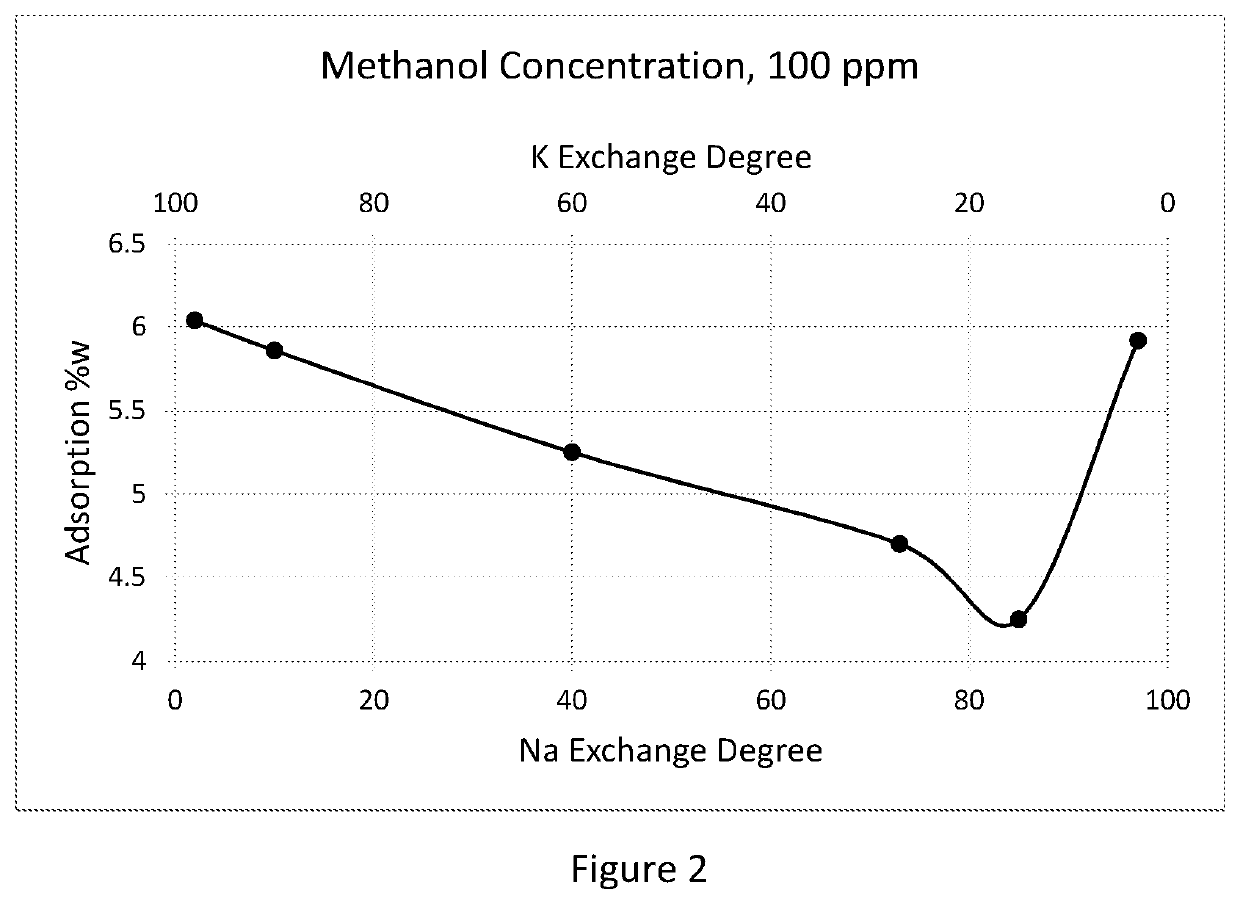
Adsorbent and process for methanol and oxygenates separation
InactiveUS20200063056A1Increase capacityHigh selectivityGas treatmentMethane captureMethacrylateAlkaline earth metal
An adsorbent separates methanol and other alcohols from gas and liquid oxygenates and hydrocarbon streams with a low silica faujasite (LSX) in a mono-, bi, or tri-cation alkali and / or alkaline-earth metal forms. The LSX has silicon to aluminum ratio from about 0.9 to about 1.15 and an ion exchange degree for each alkali or alkaline-earth metal in the range of about 10 to about 99.9% equiv. The gas streams for treatment include natural gas, individual hydrocarbons, or vaporized alkyl esters of carboxylic acids, or methyl tert-alkyl ethers and their mixtures with hydrocarbons. The liquid streams include liquefied natural gas (LNG), liquefied petroleum gas (LPG), natural gas liquid (NGL), individual hydrocarbons C3-C5, and monomers, alkyl esters of carboxylic acids including methyl acetate, methyl, ethyl, butyl acrylates and methacrylate, methyl tert-alkyl ethers including methyl tert-butyl ether (MTBE) and methyl tert-amyl ether (TAME). The adsorbent is especially suited for temperature swing or pressure swing adsorption processes.
Owner:M CHEM CO
Preparation method of macroporous strong-acidity resin and application of macroporous strong-acidity resin
ActiveCN108794662AHigh bond energyImprove thermal stabilityOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from carboxylic acid esters/lactonesCyclopenteneNitrostyrol
The invention relates to a preparation method of macroporous strong-acidity resin and application of the macroporous strong-acidity resin. The preparation method comprises the following steps: performing coreaction on p-nitrostyrene serving as a main monomer raw material, divinylbenzene serving as a crosslinking agent, a dispersing agent, an initiator and a pore-forming agent to prepare microspheres of p-nitrostyrene-divinylbenzene, and performing sulfonation reaction through chlorosulfonic acid to prepare the macroporous strong-acidity resin. The prepared macroporous strong-acidity resin hashigh heat stability and can serve as an efficient catalyst as the following system: reaction of isoamylene and methanol is catalyzed to synthesize t-amyl-methyl ether (TAME), reaction of isoamylene and ethanol is catalyzed to synthesize ethyl tert-amyl ether (ETBE), and reaction of cyclopentene and methanol is catalyzed to synthesize cyclopentyl methyl ether (CPME); and extremely high catalytic activity on reaction such as synthesis of a loxoprofen sodium precursor and loxolfonic acid is achieved. In addition, the macroporous strong-acidity resin can be recovered and reused after catalytic reaction and is a green and environmentally-friendly efficient catalytic material.
Owner:天门润诚生物科技股份有限公司
Environment-friendly gasoline additive and preparation method thereof
The invention discloses an environment-friendly gasoline additive. The environment-friendly gasoline additive is prepared from the following raw materials in parts by weight: 1-5 parts of short chainalcohol, 3-7 parts of core shell structure cerium oxide / silica particles, 2.5-7.5 parts of polyhedral oligomeric silsesquioxane, 0.5-3 parts of perfluoropolyether, 10-30 parts of ethyl tert butyl ether, 5-10 parts of diethylene glycol monomethyl amyl ether, 1-6 parts of butyl acetyl ricinoleate, and 2-8 parts of a fluorinated diamine compound. According to the environment-friendly gasoline additive, the problems such as environmental pollution, engine carbon deposition and fuel oil standing stratification generated after gasoline combustion are solved, power, anti-corrosion, anti-rust and lubrication properties of an engine are also improved, so that engine wear is reduced, the service life of the engine is prolonged, and large-scale promotion and application are more advantageous. The invention further discloses a preparation method of the environment-friendly gasoline additive.
Owner:交城县万里通加油站
Composite environmentally-friendly fuel and preparation method thereof
InactiveCN106590770AExplosion-proofNo pollution in the processLiquid carbonaceous fuelsFuel additivesIsooctyl alcoholFuran
The invention discloses a composite environmentally-friendly fuel and a preparation method thereof. The composite environmentally-friendly fuel comprises, by mass, 30-35 parts of ethylene light tar, 30-35 parts of isooctyl alcohol, 30-32 parts of dialkoxyalkane, 30-32 parts of boron trioxide, 10-12 parts of diethanolamine, 5-7 parts of di-n-amyl ether, 5-7 parts of a vinyl ester copolymer, 5-8 parts of ammonia water, 5-7 parts of furan, 5-7 parts of an antirust agent, 3-4 parts of ferrocene and 1-2 parts of camphor.
Owner:QINZHOU UNIV
Environment-friendly surface treating agent
InactiveCN108977800AStrong impact resistanceImprove adhesionMetallic material coating processesTectorial membraneWear resistant
The invention discloses an environment-friendly surface treating agent which comprises the following raw materials in parts by weight: 20-30 parts of di-n-amyl ether, 10-12 parts of octadecanol, 6-8 parts of a surface active agent, 4-7 parts of a wear-resistant anti-rust agent, 4-6 parts of a bactericide, 2-5 parts of a buffering agent, 4-6 parts of lanthanum dialkyl dithiocarbamate and 20-30 parts of water. Through optimization for the formula of surface treating agent according to the invention, the generated protective film has excellent impact resistance, the adhesive force of coating is improved, the corrosion resistance of metal surface is enhanced and the surface treating agent has safety and environmental protection property.
Owner:NANTONG KEXING CHEM
Alcohol-based fuel composite additive as well as preparation method and application thereof
The invention discloses an alcohol-based fuel composite additive as well as a preparation method and application thereof, and belongs to the technical field of fuel additives. The alcohol-based fuel composite additive comprises a base solvent, a cosolvent, a corrosion inhibitor and a water-dissolving agent, wherein the base solvent comprises isobutanol and camphor, the cosolvent comprises methyl tertiary butyl ether, ethyl tertiary butyl ether, methyl tertiary amyl ether, naphtha, xylene and petroleum ether; the corrosion inhibitor comprises benzotriazole, fatty alcohol-polyoxyethylene ether,alkylated diphenylamine, 2, 6-di-tert-butyl-4-methylphenol, N, N'-bis (salicylidene) -1, 3-diaminopropane and alkenyl succinate, wherein the water-dissolving agent comprises sorbitan trioleate, ethanol and methylal. The preparation method comprises the following steps: uniformly mixing and mixing the raw materials, the prepared alcohol-based fuel composite additive is good in stability, good in intersolubility and high in universality, has good environmental benefits, economic benefits and social benefits, and has a good market application prospect, and the preparation method is simple and issuitable for industrial popularization and application.
Owner:钱丰 +2
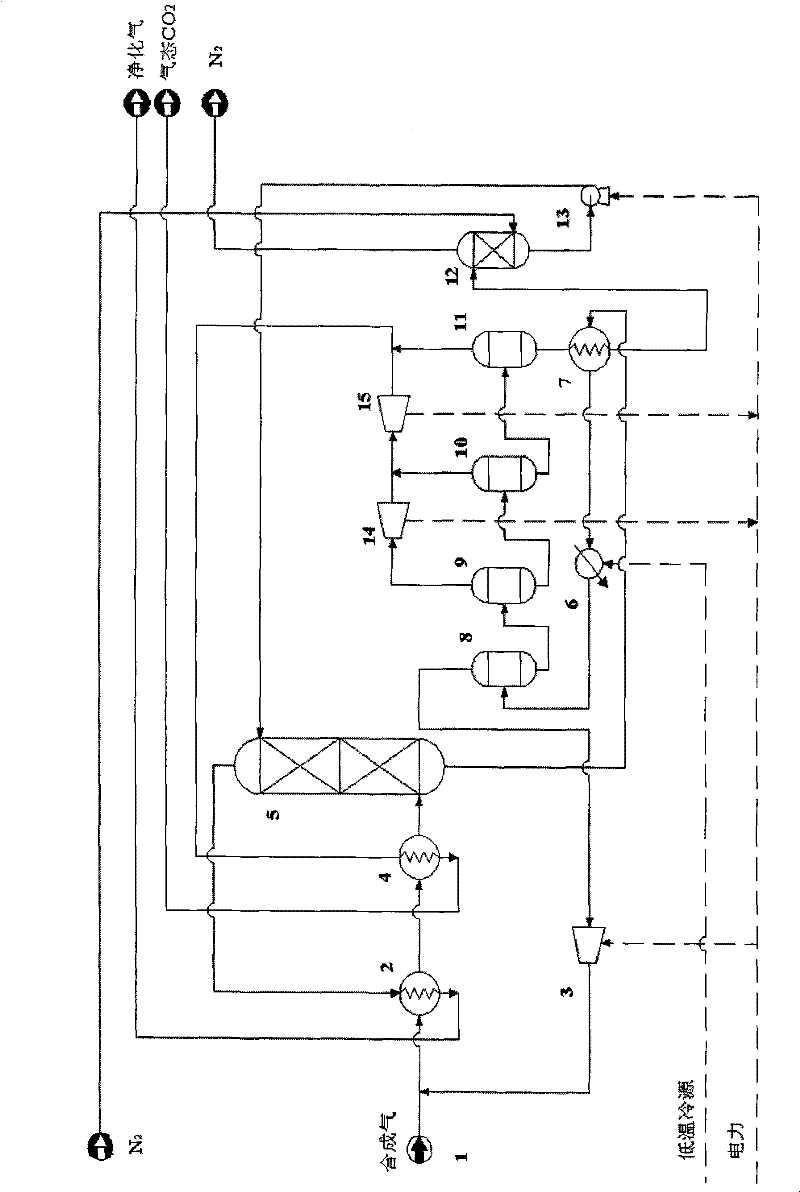
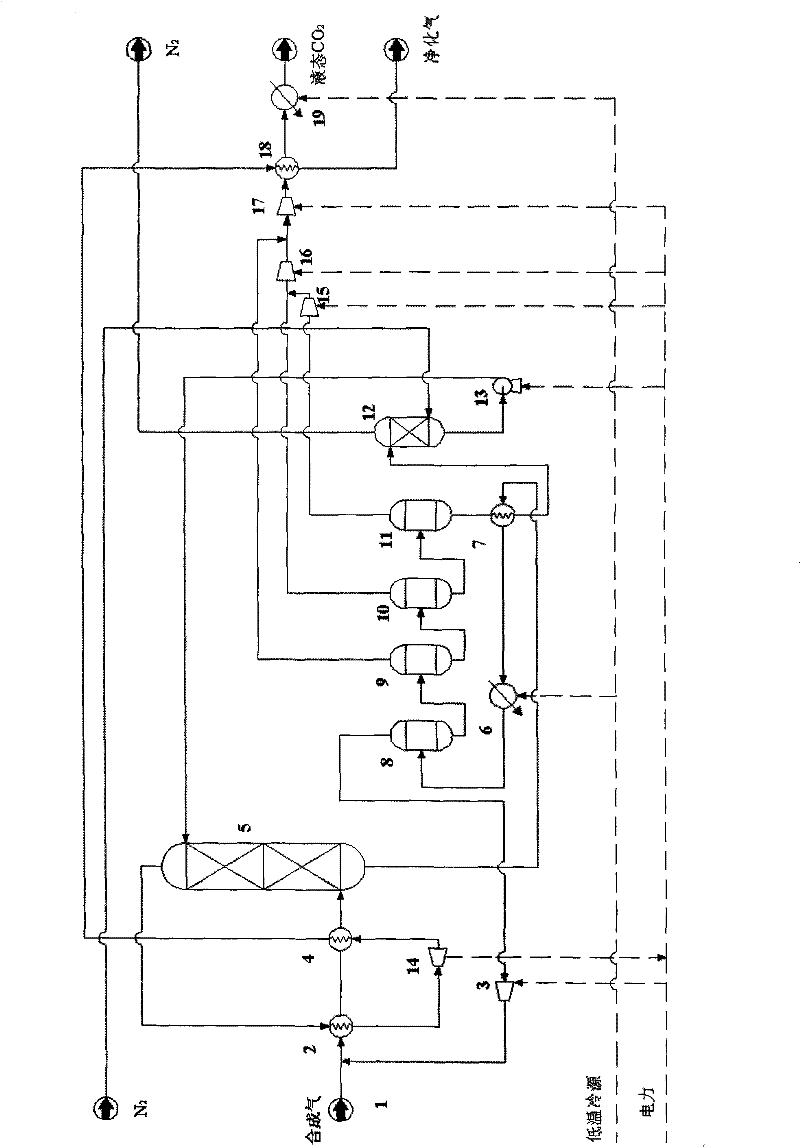
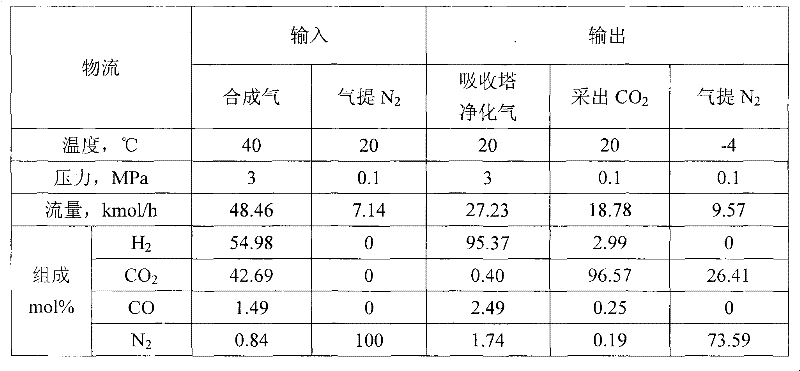
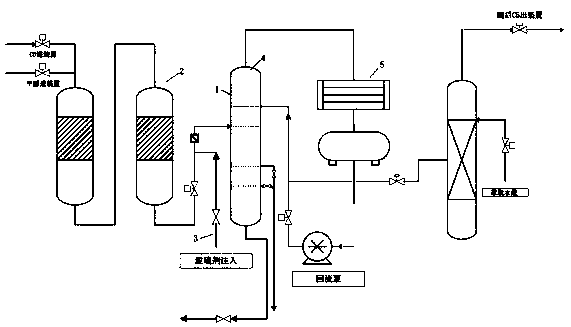
Method for separating and recycling CO2 from mixed gas containing CO2
InactiveCN101637694BImprove solubilityPromote absorptionDispersed particle separationFlash distillationEnergy recoveryMixed gas
The invention relates to a method for separating and recycling CO2 from mixed gas containing CO2, comprising a CO2 separation process and a novel absorbent, wherein, the separation process is composed by gas absorption technology, four-stage flash evaporation deabsorption technology and N2 air stripping absorbent regeneration technology, and the novel absorbent is composed by one or any two of normal or isomeric propyl ether, butyl ether and amyl ether. By adopting the absorbent and process in the invention, the content of CO2 in the purified gas can reach below 0.4%; the purity of extracted CO2 can reach over 96.5%; and the recovery rate of CO2 in the raw material mixed gas can reach over 86%. In the invention, the operating pressure of the process is normally 2.0 MPa to 4.0 MPa, and generally the virgin gas does not need to be pressurized, thus being capable of saving compression energy consumption. Cold energy recovery and excess pressure power recovery are carried out on the extracted gas flow in the invention, thus saving the separation energy consumption of CO2 and realizing the gas purification with low consumption and the liquid collecting of CO2.
Owner:BEIJING UNIV OF CHEM TECH
A kind of preparation method of methyl tert-amyl ether and a kind of upgrading method of light gasoline
ActiveCN107935821BStable conversion rateBoost octaneOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsTert-Amyl methyl etherPolymer science
The invention discloses a tert-amyl methyl ether preparation method and a light gasoline modification method. The tert-amyl methyl ether preparation method comprises that methanol and isopentene contact an etherification catalyst under an etherification reaction condition to obtain the reaction product containing tert-amyl methyl ether, wherein the etherification catalyst is a polymer supported ionic liquid catalyst, and has a structure represented by a formula (I) or a formula (II). With the method of the present inventin, the tert-amyl methyl ether preparation reaction can maintain the highreactivity.
Owner:CHINA PETROLEUM & CHEM CORP +1
A kind of purification technology of methyl tert-amyl ether purity standard substance raw material
ActiveCN109400451BHigh purityImprove product qualityEther separation/purificationTert-Amyl methyl etherPhysical chemistry
The invention provides a purification technology of methyl tert-amyl ether purity standard substance raw material, comprising steps: (1) precision rectification: under normal pressure, adding industrial-grade methyl tert-amyl ether into a rectification tower, the rectification tower Built-in packing, obtain the methyl tert-amyl ether product from the top of the rectification tower, discard the front fraction with more impurities to obtain the sample to be adsorbed; (2) adsorption: at normal temperature, transfer the sample to be adsorbed obtained in step (1) to Adsorption tower, the adsorption tower has a built-in adsorbent, and the methyl tert-amyl ether product is obtained from the bottom of the adsorption tower; (3) Filtration: at normal temperature, the methyl tert-amyl ether product obtained in step (2) is passed through an organic microporous membrane Filter to obtain high-purity methyl tert-amyl ether. The purification technology of the present invention can solve the difficulty in the prior art that various trace impurities are difficult to remove, and the obtained methyl tert-amyl ether has a purity higher than 99.9%, less impurities and can be used as a standard substance.
Owner:NAT INST OF METROLOGY CHINA
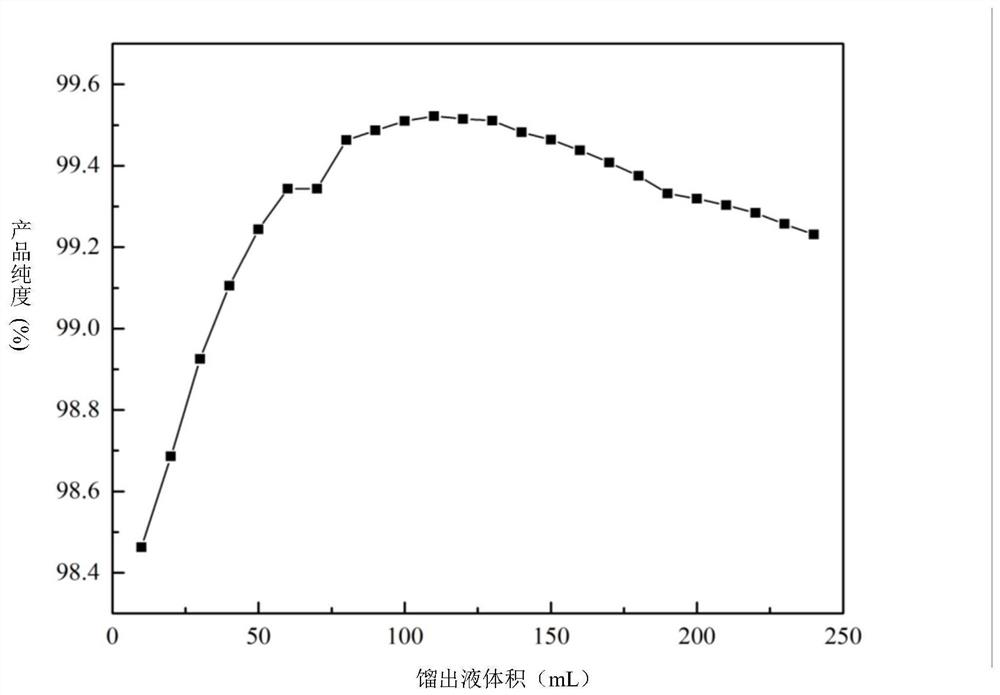
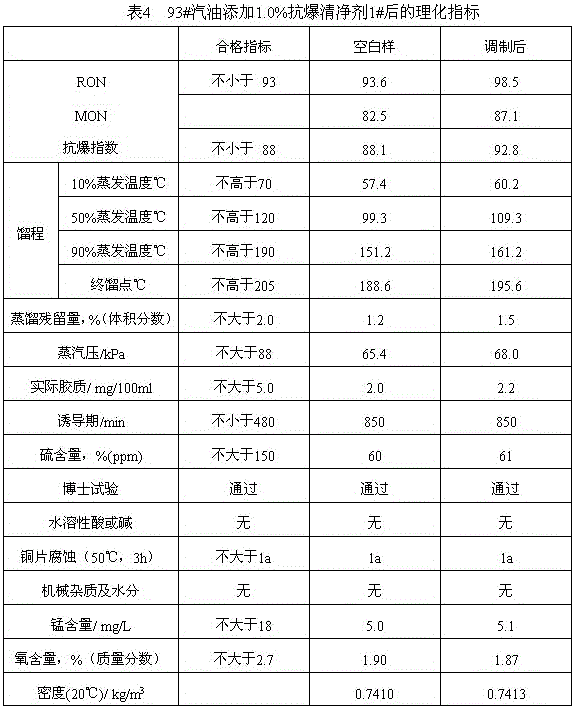
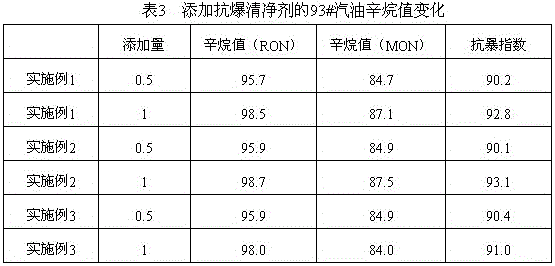
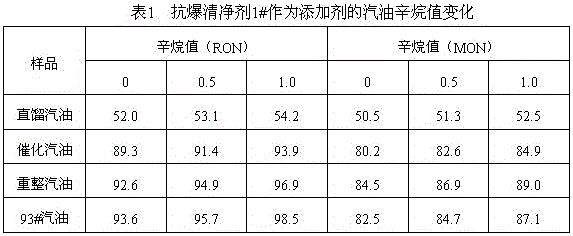
A gasoline antiknock detergent
ActiveCN104560239BBoost octaneGood environmental compatibilityLiquid carbonaceous fuelsFuel additivesDimethylaniline N-oxideGasoline
The invention provides an anti-explosion gasoline cleansing agent, which is prepared by compounding methyl amyl ether / methyl tert-butyl ether mixed liquor and dimethylaniline according to the volume ratio of 1:(1-2), wherein the methyl amyl ether / methyl tert-butyl ether mixed liquor is distillate mixed liquor of a product obtained by reacting equal volumes of raw materials tert-butyl alcohol and pentanol with methanol under the catalysis of sulfuric acid. After 0.5 to 1 weight percent of anti-explosion gasoline cleansing agent is added into gasoline, the octane value of the gasoline can be remarkably improved, and high environmental intermiscibility is achieved.
Owner:CRPC INNOVATION ENERGY
Comprehensive utilization method of mixed C4-C5 material
PendingCN114456030AIncrease added valueHigh chemical added valueHydrocarbon by isomerisationHydrocarbon by hydrogenationAlkaneMethyl t-butyl ether
The invention relates to a comprehensive utilization method of a mixed C4 and C5 material, compared with a traditional comprehensive utilization process of mixed hydrocarbon, the comprehensive utilization method provided by the invention can be used for dehydrogenation treatment of the mixed material containing C4 and C5 alkanes, components with high added values in the mixed C4 and C5 material are fully utilized, and the utilization rate of the mixed C4 and C5 material is improved. The methyl tert-butyl ether and the methyl tert-amyl ether with higher chemical additional values are directly obtained, and a better cracking furnace cracking raw material and another important chemical raw material propylene are also obtained. Therefore, each component of the mixed C4-C5 material is utilized, and the additional value of the mixed C4-C5 material is greatly improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing high-quality thioglycolic acid from the tail liquid of producing o-alkyl-n-alkylthiocarbamate
ActiveCN105254547BHigh recovery rateHigh purityOrganic chemistryOrganic compound preparationCarbamateGlutaric acid
The invention discloses a method for preparing high-quality mercaptoacetic acid from a tail solution from O-alkyl-N-alkyl thinocarbamate production. The method comprises the following steps: (1) performing acidizing treatment on the tail solution by using an inorganic acid, and controlling the pH value to be 0.5-1.5; (2) leaving to stand for 6-48 hours, separating suspended organic matters, extracting by using an organic solvent, and extracting by using an extraction agent, thereby obtaining an extraction liquid, wherein the volume ratio of the total amount of the organic solvent to an acidifying liquid is (2.5:0.01)-(2.5:0.1), the organic solvent consists of 40-60wt% of glutaric acid diethyl ester and 40-060wt% of ethyl malonic acid diethyl ester, the volume ratio of the total amount of the extracting agent to a new acidifying liquid is (2:0.1)-(2:2), and the extracting agent is prepared by mixing n-amyl ether, isoamyl ether and sec-butyl methyl ether in a mass ratio of (1-3):(1-3):(1-3); (3) removing the solvent and water from the extraction liquid, thereby obtaining crude acid; (4) performing flash evaporation on the crude acid. The method is high in recycling rate, high in product purity, good in quality and simple in process.
Owner:QINGDAO LNT CHEM
Synthesis method of oseltamivir
InactiveCN103833570BHigh yieldEasy to operateOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsAziridine
The invention discloses a synthesis method of oseltamivir. The synthesis method of oseltamivir comprises the following steps: starting from a compound 1,3-butadiene-3-amyl ether and compound 3-nitro-ethyl acrylate, carrying out Diels-Alder reaction, then reacting at room temperature in acetonitrile in the presence of a copper catalyst and PhI-NNs to prepare an aziridine compound in a one-pot method, wherein the mole ratio of the 1,3-butadiene-3-amyl ether to 3-nitro-ethyl acrylate to the copper catalyst is 1.1: 1: 0.025-0.1; and finally synthesizing the oseltamivir for preventing bird flu through the aziridine ring opening, nitryl and p-nitrobenzene sulfonyl removal, acetylation and hydrogenation. The method comprises short steps, the used reagent is cheap and easily available, the operation is simple, the total yield is up to 40%, and the method is a simple and efficient synthesis method of oseltamivir.
Owner:ZHEJIANG NORMAL UNIVERSITY
A modification method of sulfonic acid-based cation exchange resin and a method for preparing methyl tert-amyl ether
ActiveCN107537567BHigher than the surfaceHigh pore volumeOrganic-compounds/hydrides/coordination-complexes catalystsEther preparation by compound additionTert-Amyl methyl etherPolymer science
The invention discloses a method for modifying a sulfonic cation exchange resin, and a methyl tert-amyl ether preparation method. According to the present invention, a sulfonic cation exchange resin is soaked in a low-carbon alcohol solvent, and the soaked sulfonic cation exchange resin is subjected to gas stripping treatment with an inert gas, such that the specific surface area and the pore sizestructure of the resin are improved, and the stability and the selectivity of the sulfonic cation exchange resin catalyst are improved; and with the application of the modified resin as the catalystin the preparation of methyl tert-amyl ether at a temperature of 80-160 DEG C under a pressure of 0.1-3.0 MPa by using C5 olefin and methanol as raw materials, the continuous production can be achieved, the advantages of high conversion rate, good stability and the like can be provided, and the method is the new environmentally friendly process.
Owner:CHINA PETROLEUM & CHEM CORP +1
A modification method of cation exchange resin and method for preparing methyl tert-amyl ether
ActiveCN107537568BHigher than the surfaceHigh pore volumeOrganic-compounds/hydrides/coordination-complexes catalystsEther preparation by compound additionPolymer sciencePtru catalyst
The invention discloses a cation exchange resin modification method and a methyl tert-amyl ether preparation method. According to the present invention, a sulfonic cation exchange resin is soaked sequentially with toluene and methyl isobutyl ketone, and soaking in stages is performed with distilled water, such that the specific surface and the pore size structure of the resin can be improved, andthe stability and the selectivity of the sulfonic cation exchange resin catalyst can be improved; and with the application of the modified resin as the catalyst in the preparation of methyl tert-amylether at a temperature of 75-250 DEG C under a pressure of 0.5-3.0 MPa by using C5 olefin and methanol as raw materials, the continuous production can be achieved, the advantages of high conversion rate, good stability and the like can be provided, and the method is the new environmentally friendly process.
Owner:CHINA PETROLEUM & CHEM CORP +1
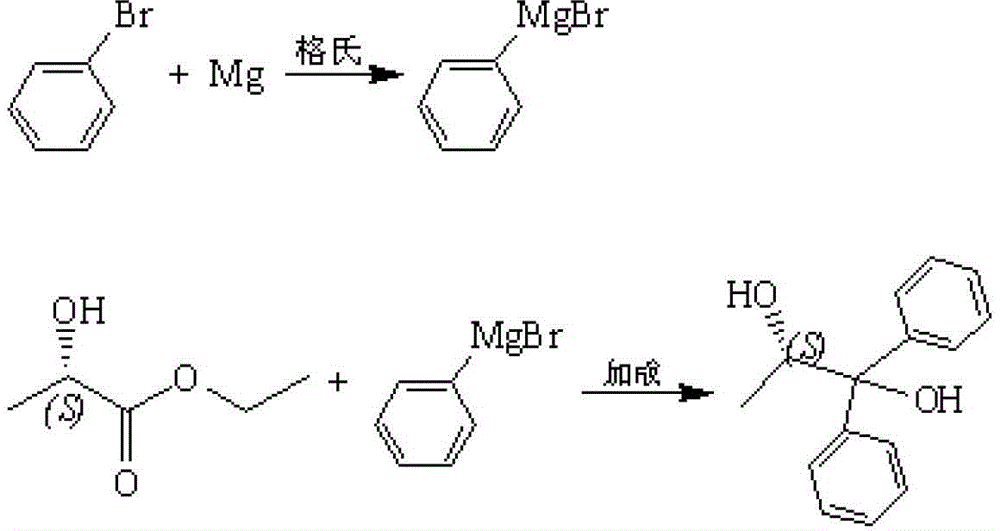
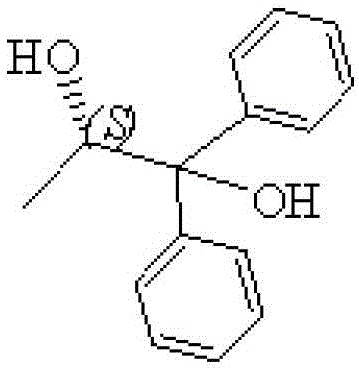
Preparation method for S-(-)-1,1-diphenyl-1,2-propylene glycol
ActiveCN103360217BGood choiceRaw materials are easy to getOrganic compound preparationHydroxy compound preparationOrganic synthesisGrignard reagent
The invention provides a preparation method for S-(-)-1,1-diphenyl-1,2-propylene glycol, which belongs to the field of organic synthesis. The method comprises the following steps: with bromobenzene and magnesium as raw materials and iodine as a catalyst, preparing a Grignard reagent in an organic solvent through a Grignard reaction; with ethyl lactate as a raw material, subjecting ethyl lactate and the Grignard reagent to an addition reaction in the organic solvent under the protection of nitrogen; adding water after the reaction is finished, adjusting a pH value with inorganic acid, carrying out extraction and layering and collecting an organic layer for concentration; and carrying out recrystallization in an organic dissolvent so as to obtain S-(-)-1,1-diphenyl-1,2-propylene glycol. The organic solvent used in the method is an ether, namely, one selected from the group consisting of diethyl ether, butyl ether, amyl ether, tetrahydrofuran and dioxane, reaction temperature is 20 to 70 DEG C, and reaction time is 2 to 6 h, and a mol ratio of bromobenzene to magnesium to iodine is 1: 0.9-1.2: 0.001-0.002. According to the invention, the raw materials are easily available, cost is low, operation in reaction process is clear, the product S-(-)-1,1-diphenyl-1,2-propylene glycol has good purity and higher yield, the method is suitable for industrial production, and production efficiency is improved.
Owner:NORTHEAST PHARMA GRP
C5 desulfurization purification process
InactiveCN110746260AHigh purityLow purityEther separation/purificationChemical industryTert-Amyl methyl etherProcess engineering
The invention relates to a C5 desulfurization purification process. A pipe static mixer is additionally arranged in front of material feeding of a C5 catalytic distillation tower, a desulfurizer injection port is additionally arranged in front of the pipe static mixer, and the desulfurizer injection port is connected with a metering pump. A gas phase extraction outlet is arranged in the middle andupper part of the C5 catalytic distillation tower, and through the gas phase extraction outlet, methyl tert-amyl ether in the C5 catalytic distillation tower is extracted. Through technical reform ofthe process, the extraction way of methyl tert-amyl ether is changed, by adding a desulfurizer at the desulfurizer injection port, the total sulfur content in the methyl tert-amyl ether and C5 can bereduced to no greater than 10PPM, the purity of C5 is increased to 95% or greater, the investment is small, the energy consumption is low, effects can be taken into play rapidly, long-term quality stability can be maintained, the market competitiveness can be improved, and benefits can be brought to enterprises.
Owner:宁波科元精化股份有限公司
A kind of rare earth modified gasoline additive and its preparation method
InactiveCN110373236BIncrease surface areaBurn fullyLiquid carbonaceous fuelsCerium nitratePolythylene glycol
The invention discloses a preparation method of a rare earth modified gasoline additive. S1: Co-precipitate the lanthanum nitrate, cerium nitrate and cobalt nitrate solution by adding sodium hydroxide dropwise to obtain a precursor solution; calcinate at a high temperature to obtain La / Ce / Co ternary composite oxide; S2: disperse it in polyvinylpyrrolidone, add a surface modifier to obtain a surface-modified rare earth composite oxide; S3: weigh short-chain alcohol, perfluoropolyether by weight , ethyl tert-butyl ether, dipolyethylene glycol monomethyl pentyl ether, butyl acetyl ricinoleic acid ester, fluorine-containing diamine compound; after mixing evenly, add rare earth composite oxide and cage-type silsesquisil oxane to obtain the rare earth modified gasoline additive. The rare-earth modified gasoline additive prepared by the invention not only solves the problems of engine carbon deposition and fuel stratification, but also improves the overall performance of the engine, prolongs the service life of the engine, and has a huge market application prospect.
Owner:杭州碧源节能科技有限公司
A method for comprehensive utilization of olefins in Fischer-Tropsch synthesis light distillate oil
ActiveCN104370678BSolve problems that need to be exploitedAlleviate the contradiction between supply and demandDistillation purification/separationEther preparation by compound additionIsomerizationMeth-
The invention relates to a comprehensive processing method for olefins in Fischer-Tropsch synthesis light distillate oil. The high-temperature Fischer-Tropsch synthesis C5 light distillate oil is used as raw material, and the raw material is extracted and rectified, and the extractant is N, N-dimethyl formazan Amide, the enriched 1-pentene material obtained at the top of the extractive distillation tower, is further purified by precision rectification to obtain 1-pentene product, and the C5 component and extractant mixture obtained at the bottom of the tower enter the solvent recovery tower for recovery Extractant for recycling; the carbon five components obtained from the top of the solvent recovery tower are subjected to olefin isomerization through the isomerization reactor; the carbon five components rich in isomerized olefins obtained from the isomerization reactor are mixed with methanol enter the etherification reactor together for etherification reaction; the outlet material of the etherification reactor enters the catalytic rectification tower, and realizes the separation of the product methyl tert-amyl ether while carrying out the etherification reaction in the catalytic rectification tower. At the bottom of the distillation tower, the industrially required methyl tert-amyl ether product is obtained. The invention obtains high value-added carbon pentaolefin 1-pentene and clean high-octane gasoline additive methyl tert-amyl ether through deep processing of Fischer-Tropsch carbon five distillate oil.
Owner:TAIYUAN UNIV OF TECH
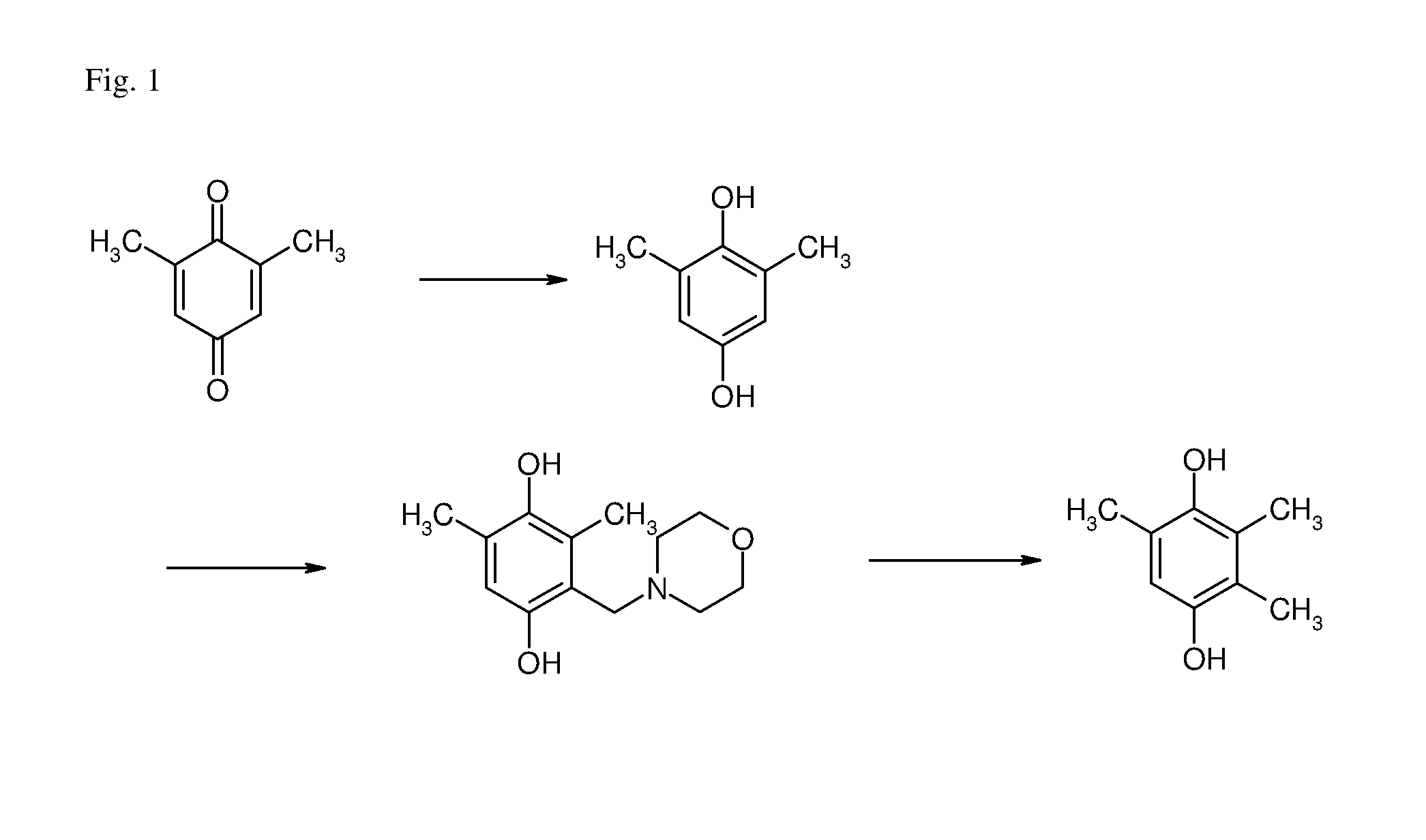
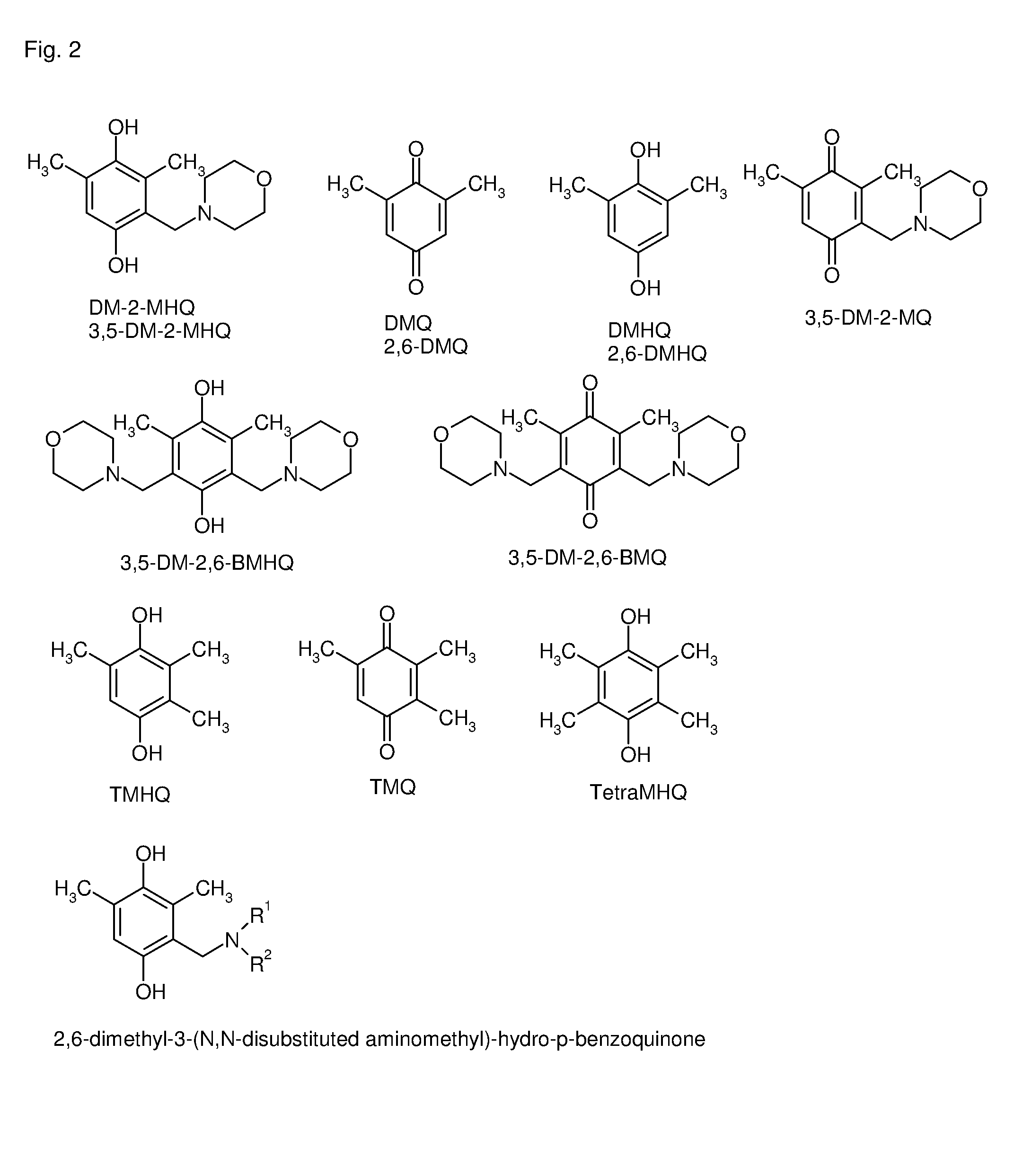
Process for the manufacture of TMHQ
ActiveUS8809591B2Good yieldOrganic compound preparationCarbonyl compound preparationHydrogenOrganic solvent
The present invention is directed to a process for the manufacture of 2,3,5-trimethyl-hydro-p-benzoquinone comprising the following steps: a) hydrogenating 2,6-dimethyl-p-benzoquinone with hydrogen in the presence of a hydrogenation catalyst in an organic solvent to obtain 2,6-dimethyl-hydro-p-benzoquinone; b) reacting 2,6-dimethyl-hydro-p-benzoquinone with a secondary amine and formal-dehyde in an organic solvent to obtain 2,6-dimethyl-3-(N,N-disubstituted aminomethyl)-hydro-p-benzoquinone; c) reacting 2,6-dimethyl-3-(N,N-disubstituted aminomethyl)-hydro-p-benzoquinone with hydrogen in the presence of a hydrogenolysis catalyst in an organic solvent to obtain 2,3,5-trimethylhydro-p-benzoquinone; wherein the organic solvent in all steps a), b) and c) is independently selected from the group consisting of methyl tert.-butyl ether, ethyl tert.-butyl ether, methyl tert.-amyl ether, methoxycyclopentane and any mixtures thereof. Preferably the organic solvent used in all steps a), b) and c) is the same.
Owner:DSM IP ASSETS BV
Anti-explosion gasoline cleansing agent
ActiveCN104560239ABoost octaneGood environmental compatibilityLiquid carbonaceous fuelsFuel additivesDimethylaniline N-oxideGasoline
The invention provides an anti-explosion gasoline cleansing agent, which is prepared by compounding methyl amyl ether / methyl tert-butyl ether mixed liquor and dimethylaniline according to the volume ratio of 1:(1-2), wherein the methyl amyl ether / methyl tert-butyl ether mixed liquor is distillate mixed liquor of a product obtained by reacting equal volumes of raw materials tert-butyl alcohol and pentanol with methanol under the catalysis of sulfuric acid. After 0.5 to 1 weight percent of anti-explosion gasoline cleansing agent is added into gasoline, the octane value of the gasoline can be remarkably improved, and high environmental intermiscibility is achieved.
Owner:CRPC INNOVATION ENERGY
A kind of preparation method of methyl tert-amyl ether
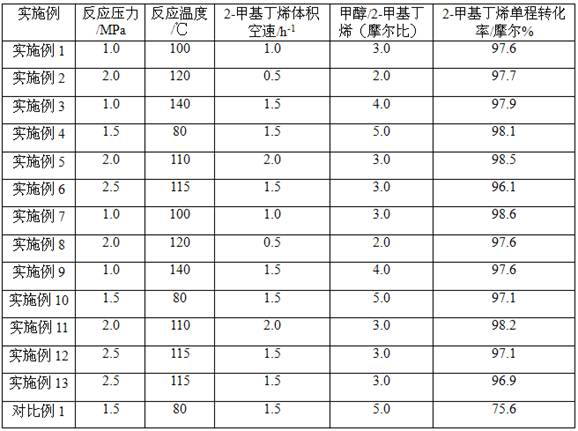
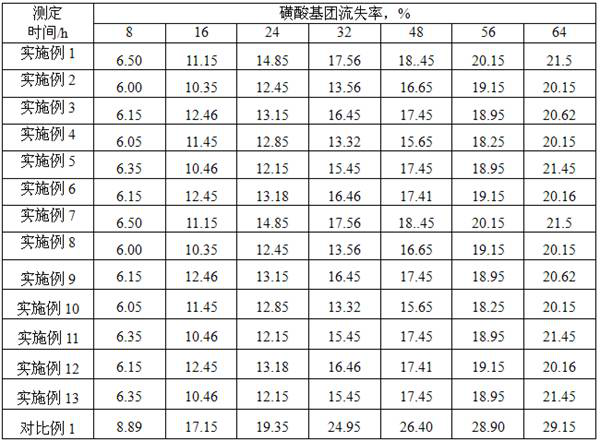
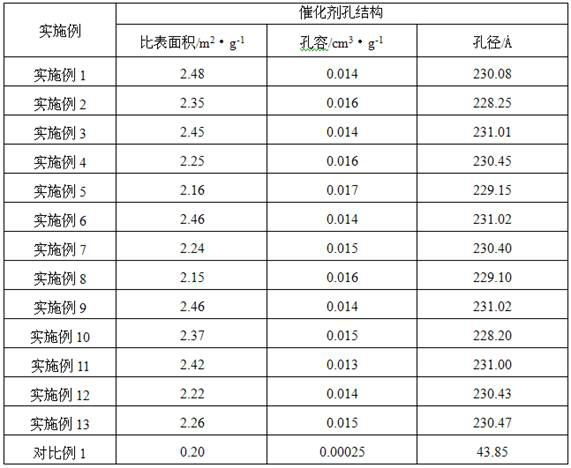
ActiveCN107540524BHigher than the surfaceHigh pore volumeEther preparation by compound additionPolymer sciencePtru catalyst
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com