A kind of preparation method of methyl tert-amyl ether and a kind of upgrading method of light gasoline
A technology of methyl tert-amyl ether and light gasoline, which is applied in the fields of ether preparation, chemical instruments and methods, addition of unsaturated compounds to ether, etc., which can solve the problems of low catalytic activity, easy falling off of catalyst sulfonic acid groups, and catalyst heat loss. Low stability and other issues, to achieve the effect of high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] According to the present invention, the preparation method of described methyl tert-amyl ether comprises:
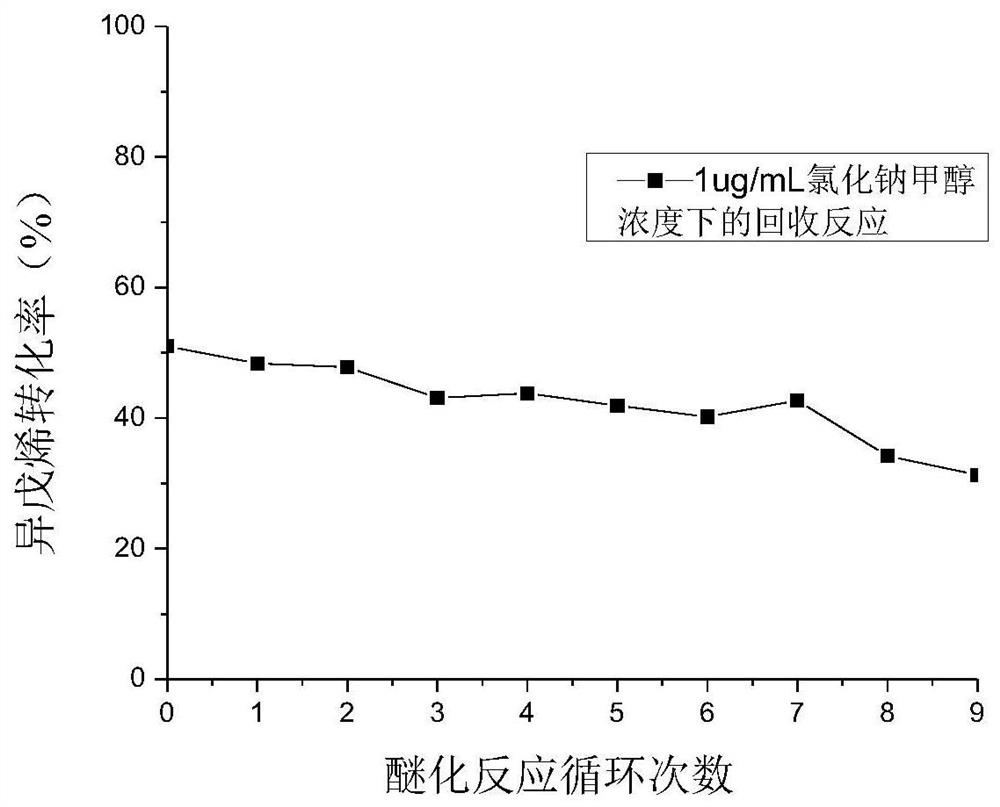
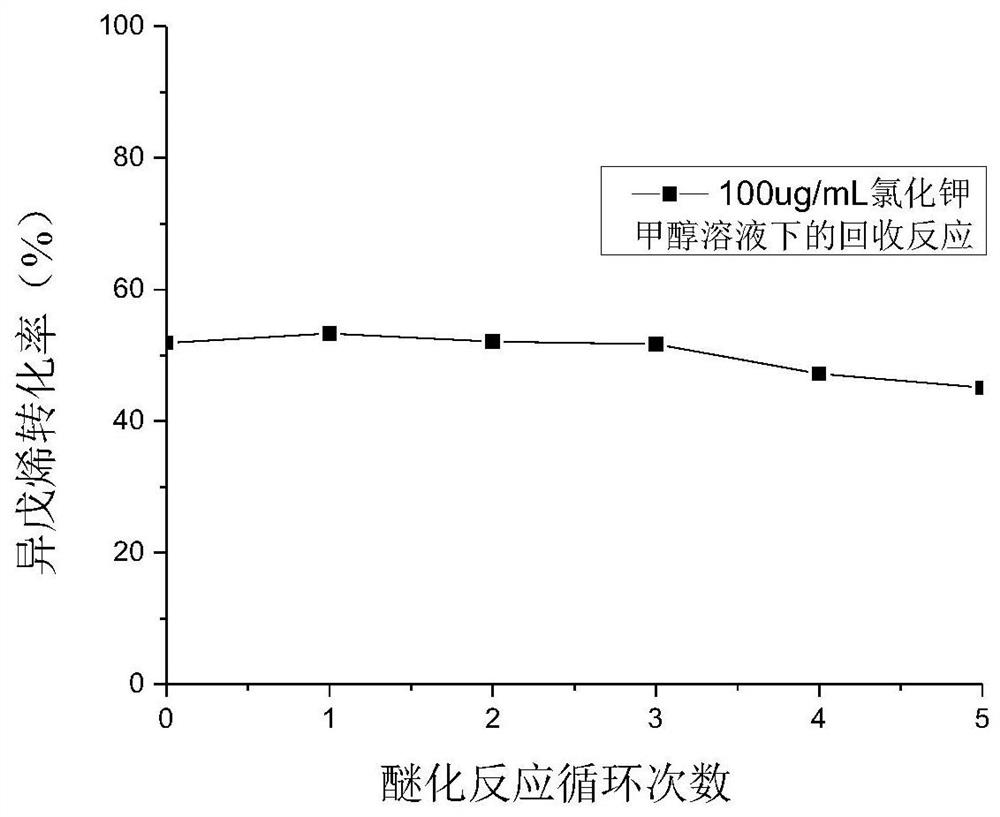
[0027] Under etherification reaction conditions, methanol and isopentene are contacted with an etherification catalyst to obtain a reaction product containing methyl tert-amyl ether;
[0028] Described etherification catalyst is polymer supported ionic liquid catalyst, and this catalyst has the structure shown in formula (I) or formula (P):
[0029]
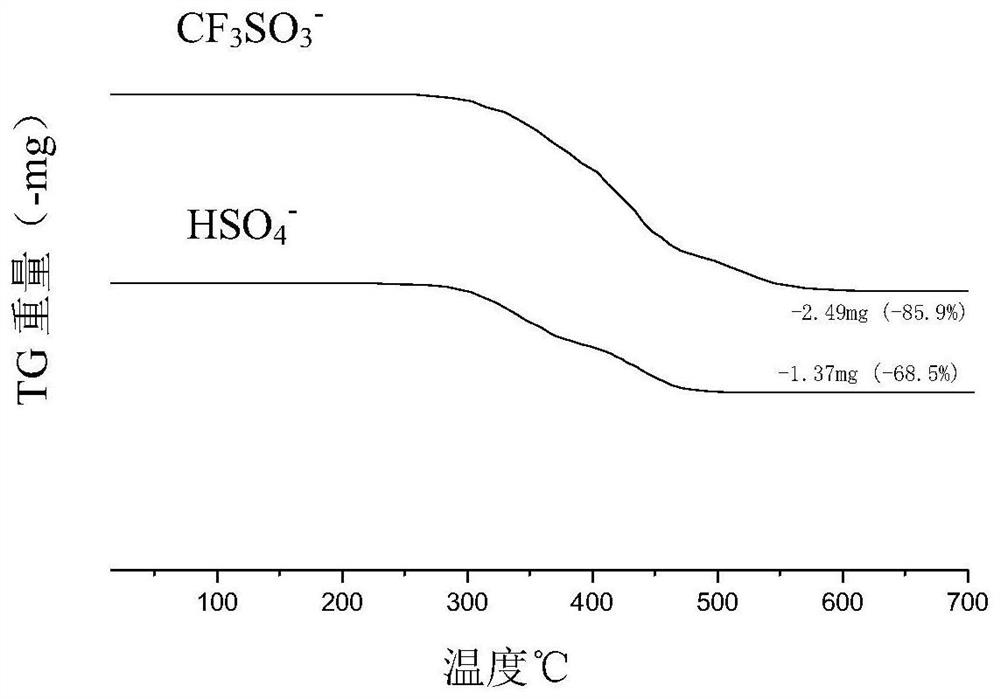
[0030]In formula (I), m is an integer of 50-200, n1 and n2 are each independently an integer of 30-100, X - from HSO 4 - 、CF 3 SO 3 - 、CH 3 SO 3 - and p-CH 3 C 6 h 4 SO 3 - one or more of
[0031] Preferably, in formula (I), m is an integer of 50-150, n1 and n2 are each independently an integer of 30-70, and X - for HSO 4 - or CF 3 SO 3 - ;
[0032] Preferably, in formula (I), n1:n2 is 1:0.9-1.1.
[0033] In formula (П), m' is an integer of 50-200, n1' and n2' are each independently an intege...
preparation Embodiment 1
[0101] This preparation example is used to illustrate the preparation of etherification catalyst.
[0102] Add 527mg chain transfer reagent CPBD (2.385mmol) and 130mg AIBN (0.795mmol) to a 100mL Schlenk bottle first, then add 39.7g styrene, close the system, pump air for 10min after freezing in liquid nitrogen, thaw in cold water with nitrogen, repeat Refrigerate and ventilate three times, put the thawed Schlenk bottle into an oil bath preheated to 80°C, and polymerize for 11 hours. The reaction was quenched with liquid nitrogen, and the crude product was analyzed by NMR after thawing at room temperature, and the conversion rate of styrene was calculated to be 56.7%. Dilute the reaction solution with THF, and reprecipitate three times in anhydrous methanol to obtain a pink powder polystyrene PS 91 18.483 g.
[0103] Add 10 g of PS to a 100 mL Schlenk tube 91 (1.032mmol), 56mg AIBN (0.344mmol) and 13.087g of 4-vinylpyridine (123.84mmol), then add 15mL DMF and stir to dissol...
preparation Embodiment 2
[0107] This preparation example is used to illustrate the preparation of etherification catalyst.
[0108] Prepare the etherification catalyst according to the method of Preparation Example 1, the difference is: in the step of loading the acidic active center on the zwitterionic catalyst precursor, add the catalyst precursor PS 91-46-47 2g (0.067mmol ), install a constant pressure dropping funnel and a reflux condensing device, close the system, pump and change the air three times, add 40mL of dry dichloromethane under nitrogen protection, put it into an oil bath preheated to 40°C, stir for 1h, and then add dropwise 2.8ml (31.47mmol) of trifluoromethanesulfonic acid, stirred and reacted for 24h, reprecipitated the reaction solution in anhydrous ether, filtered with suction, and dried in vacuum to obtain 1.83g of catalyst PS91-46-47CF 3 SO 3 - .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com