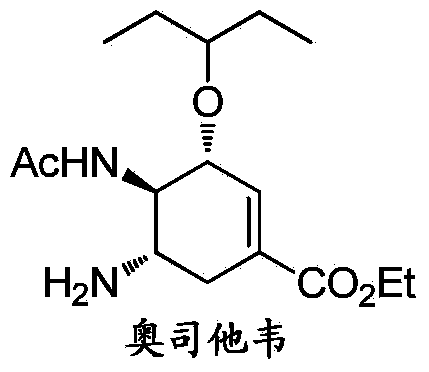
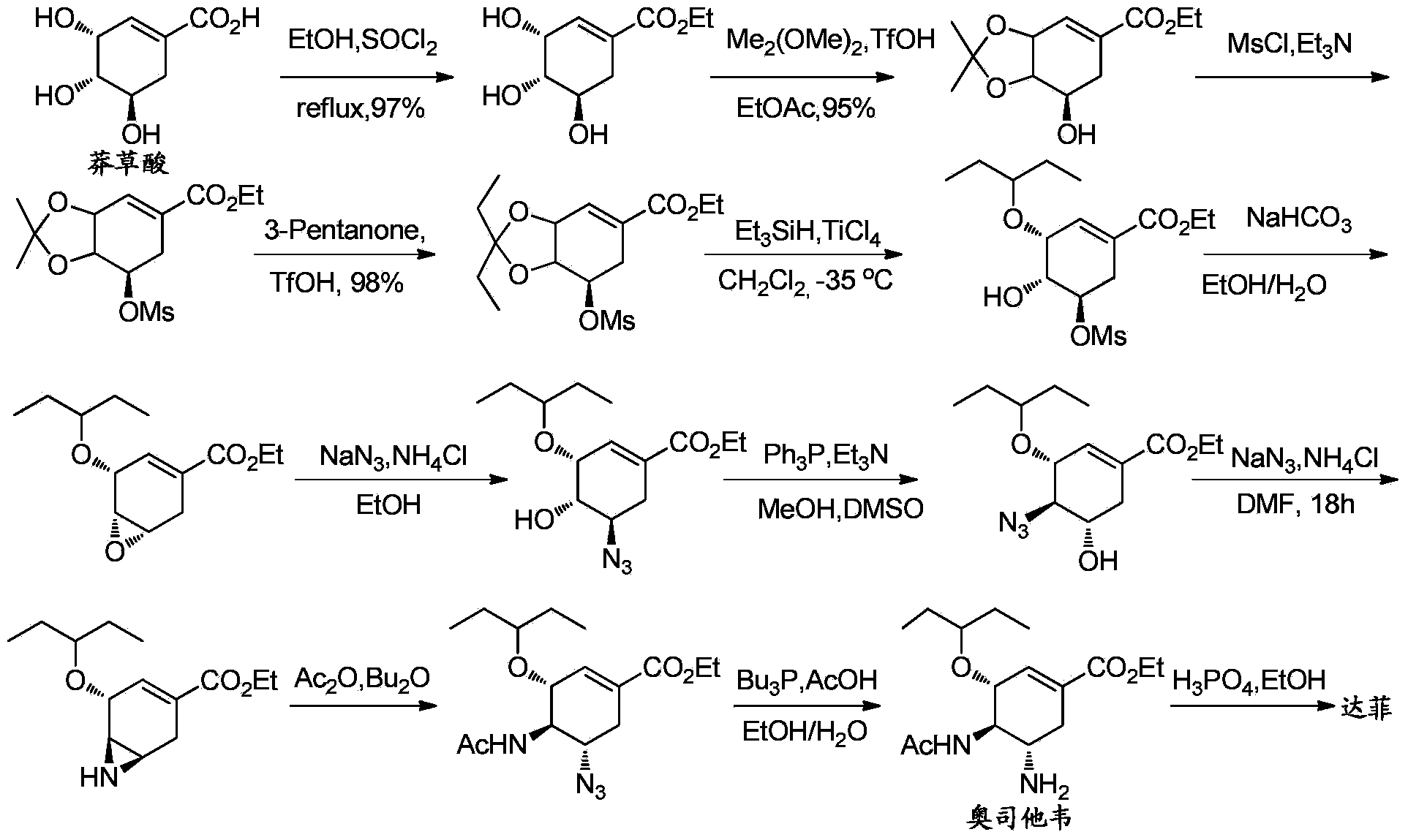
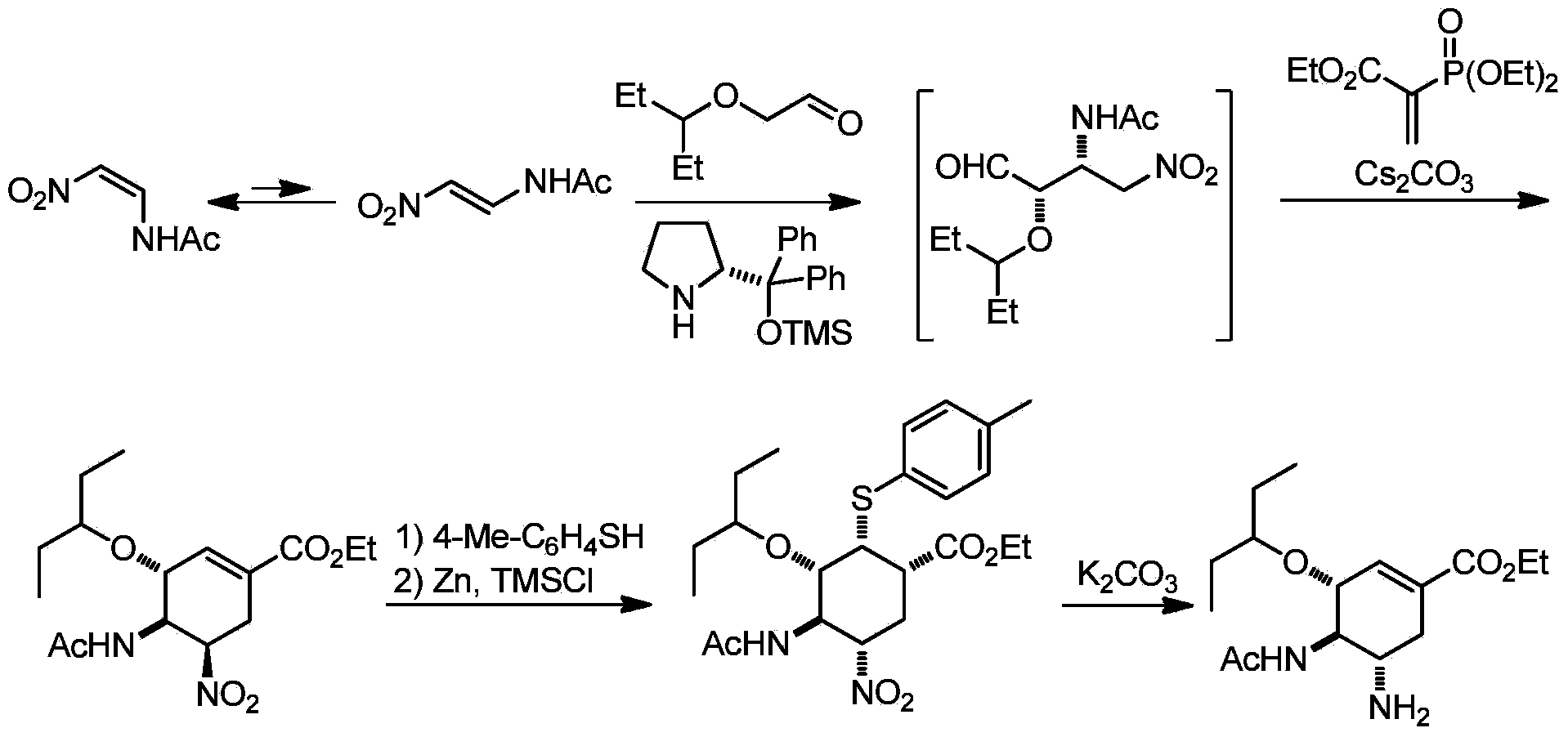
Synthesis method of oseltamivir
A synthesis method and compound technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., to achieve the effects of simple operation, cheap and easy-to-obtain reagents, and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In a 50mL round-bottom flask, add the compound represented by structural formula I (1.54g, 11mmol) and the compound represented by structural formula II (1.5g, 10mmol), heat to 70℃ and react for 4 hours, then cool to room temperature and add Cu(OTf) 2 (181mg, 0.5mmol), PhI=NNs (4.2g, 10mmol), add 20mL of acetonitrile to dissolve, stir the reaction for 6 hours, stop the reaction, remove the solvent by rotary evaporation, and separate with silica gel (300-400 mesh) column chromatography. 3.2g of colored liquid, 67% yield. Colorless liquid 1 H-NMR(CDCl 3 ,600MHz): δ0.75(t,J=7.3Hz,3H),0.91(t,J=7.3Hz3H),1.23(t,J=7.1Hz,3H),1.35-1.39(m,2H),1.49 -1.53(m,2H),1.80-1.85(m,1H),2.56-2.60(m,1H),3.21-3.24(m,1H),3.26-3.30(m,1H),3.34-3.37(m, 1H), 3.40-3.42 (m, 1H), 4.11-4.20 (m, 2H), 4.61 (s, 1H), 4.78 (dd, J=11.4, 3.0Hz, 1H), 8.16 (d, J=9.2Hz) ,2H),8.45(d,J=8.4Hz,2H); 13 C-NMR(CDCl 3 ,150MHz): δ8.8,9.5,14.0,25.0,25.2,25.9,35.9,38.8,42.6,61.7,70.5,82.7,83.4,124.7,129.2,143.4,150.9,172.1...
Embodiment 2
[0045] Except that 50°C was used instead of 70°C in Example 1 to carry out the Diels-Alder reaction of the compound represented by structural formula I and the compound represented by structural formula II, the rest of the operation steps were the same as in Example 1, to obtain the total production of oseltamivir The rate was 13%, and the product characterization data were the same as in Example 1.
Embodiment 3
[0047] Except that 90°C was used instead of 70°C in Example 1 to carry out the Diels-Alder reaction of the compound represented by structural formula I and the compound represented by structural formula II, the rest of the operation steps were the same as in Example 1, to obtain the total production of oseltamivir The rate was 31%, and the product characterization data were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com