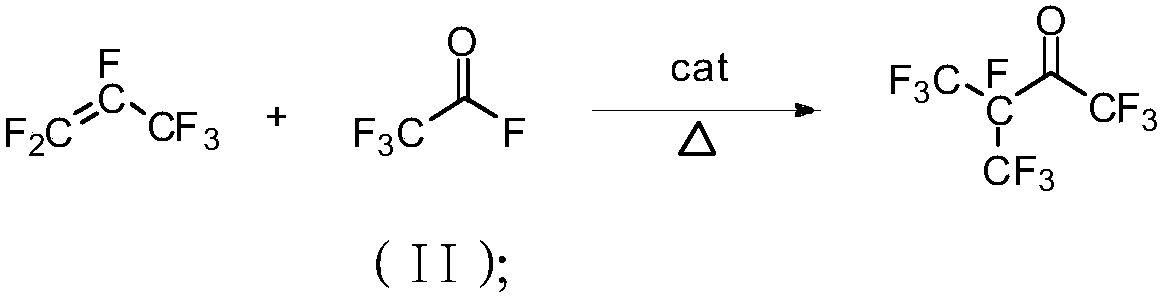Method for cracking hexafluoropropylene dipolymer to prepare perfluoro amyl ether and perfluoro amyl ether
A technology of hexafluoropropylene dimer and perfluoroamyl ether, which is applied in the field of perfluoroamyl ether, can solve the problems of less application, more by-products, large power consumption, etc., and achieves simple process operation and high conversion efficiency , the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
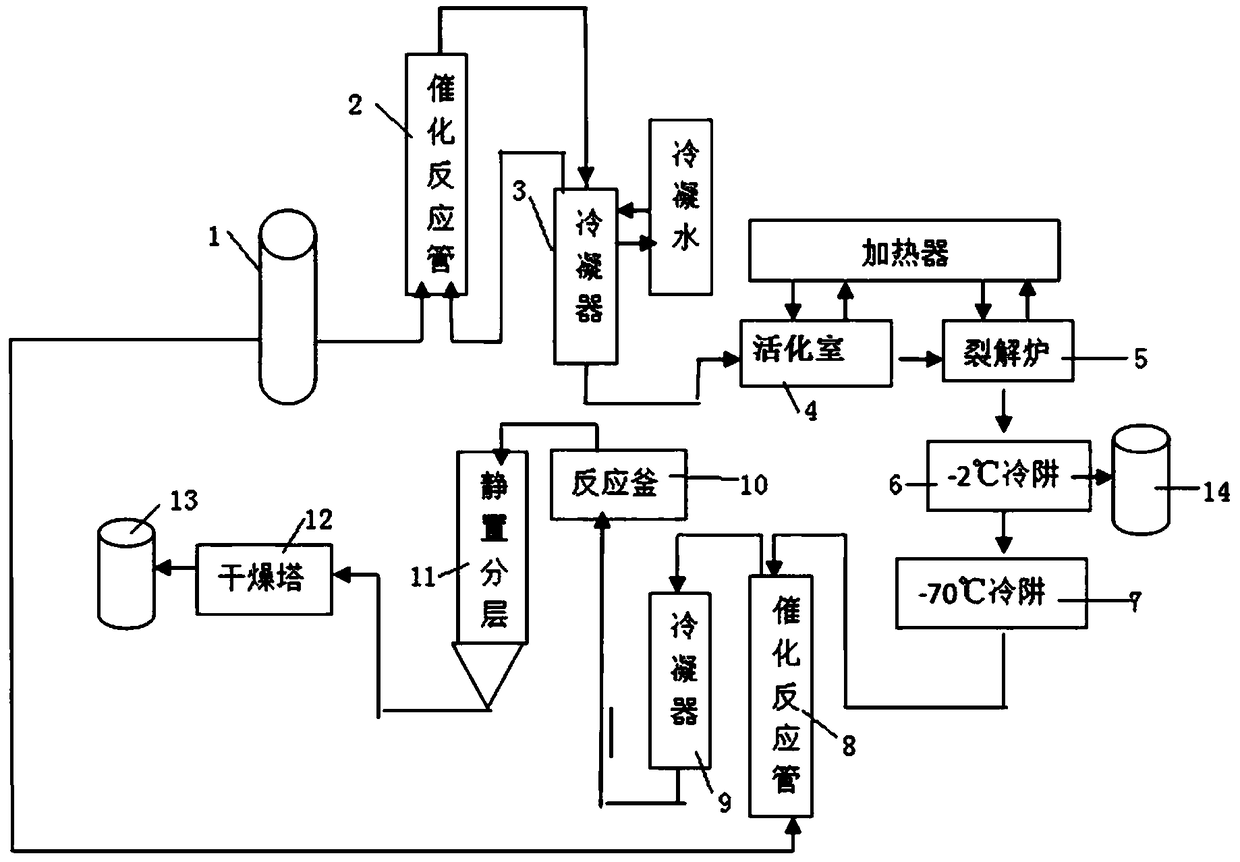
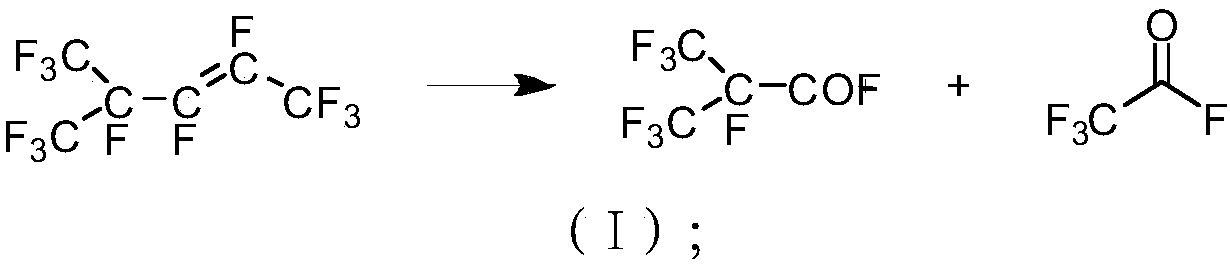
[0036] Embodiment 1: Preparation of hexafluoropropylene dimer raw material: Hexafluoropropylene gas is passed through the catalytic reaction tube equipped with NaF / Al2O3 catalyst, the loading capacity of the catalyst is 10%, and the contact time is 0.1s; the temperature of the catalytic reaction tube The temperature is 150°C. The reacted mixed gas enters the condenser with a cooling jacket from the gas port inlet. The temperature of the condensed water is 10°C. The unreacted hexafluoropropylene is circulated to the catalytic reaction tube through the gas outlet. The purity of the liquid hexafluoropropylene dimer in the receiver of the condenser was detected to be 99.3%. The yield of hexafluoropropylene dimer is shown in Table 1.
Embodiment 2
[0037] Example 2: Preparation of raw materials for hexafluoropropylene dimer: Hexafluoropropylene gas is passed through a catalytic reaction tube equipped with an AgF / C catalyst, the loading capacity of the catalyst is 15%, the temperature of the catalyst packed column is 180°C, and the contact time is For 10s, the reacted mixed gas enters the condenser with a cooling jacket through the air inlet, and the temperature of the condensed water is 10°C; the unreacted hexafluoropropylene is circulated to the catalytic reaction column through the outlet. The purity of the liquid hexafluoropropylene dimer in the condenser was detected to be 99.2%. The productive rate of hexafluoropropylene dimer after applying mechanically is shown in Table 1.
Embodiment 3
[0038] Embodiment 3: the preparation of hexafluoropropylene dimer raw material: hexafluoropropylene gas is passed through the catalytic reaction tube that KF / C catalyst is housed, and the loading capacity of catalyst is 20%, and the temperature of catalyst packing column is 220 ℃, contact time The reaction time is 30s; the reacted mixed gas enters the condenser with a cooling jacket through the air inlet, the temperature of the condensed water is 10°C, and the unreacted hexafluoropropylene is circulated to the catalytic reaction tube through the outlet. The purity of the liquid hexafluoropropylene dimer in the condenser was detected to be 99.3%. The productive rate of hexafluoropropylene dimer after applying mechanically is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com