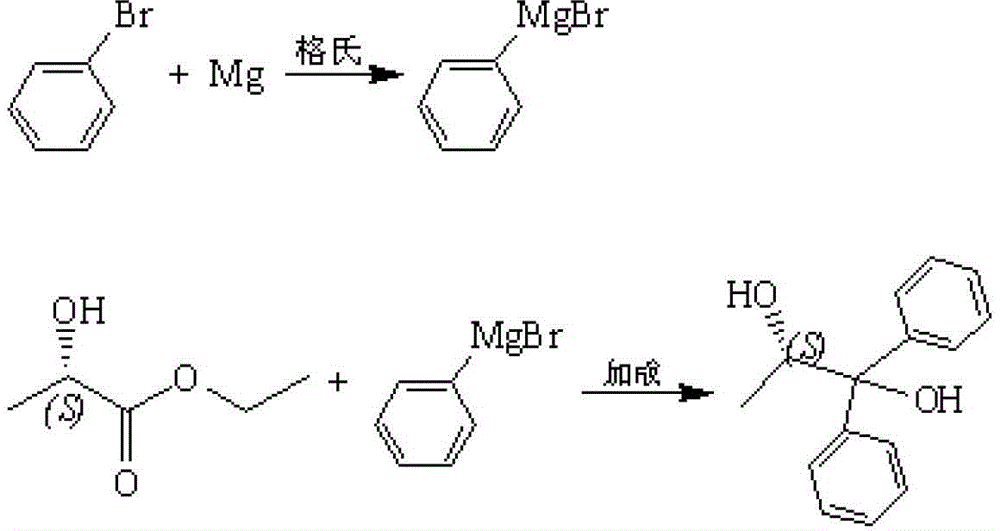
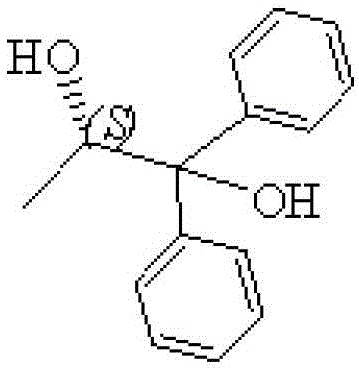
Preparation method for S-(-)-1,1-diphenyl-1,2-propylene glycol
A technology of propylene glycol and diphenyl, which is applied in the field of organic synthesis, can solve the problems of low yield, long reaction cycle, and low optical purity of products, and achieve the effects of high yield, low cost, and good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1. Preparation of formatting reagents
[0017] Prepare a mixed solution of 170g (1.08mol) of bromobenzene and 200ml of tetrahydrofuran and shake well for later use. Put 27g (1.11mol) of magnesium and 200ml of tetrahydrofuran into the reaction flask, stir, and add 0.4g (0.0016mol) of iodine. Transfer the prepared bromobenzene-tetrahydrofuran mixture to a constant pressure dropping funnel, add 10-30ml of bromobenzene-tetrahydrofuran mixture dropwise into the reaction vessel, raise the temperature to 35°C, trigger the Grignard reaction, and start adding the remaining bromobenzene - Tetrahydrofuran solution, keeping the reaction temperature not lower than 60°C. After the dropwise addition, keep the reaction for 4 hours, transfer the device to a low-temperature bath, add nitrogen protection, and set aside.
[0018] 2. Preparation of S-(-)-1,1-diphenyl-1,2-propanediol
[0019] Prepare a mixed solution of 34g (0.29mol) of ethyl lactate and 150mL of tetrahydrofuran. Lower t...
Embodiment 2
[0021] 1. Preparation of formatting reagents
[0022] Prepare a mixed solution of 170g (1.08mol) bromobenzene and 200ml ether and shake well for later use. Put 24g (0.99mol) of magnesium and 200ml of ether into the reaction flask, stir, and add 0.3g (0.0012mol) of iodine. Transfer the prepared bromobenzene-ether mixture to a constant pressure dropping funnel, add 10-30ml of bromobenzene-ether mixture dropwise into the reaction vessel, raise the temperature to 30°C, trigger the Grignard reaction, and start adding the remaining bromobenzene - Diethyl ether solution, keeping the reaction temperature not lower than 65°C. After the dropwise addition, keep the temperature for reaction for 3 hours, transfer the device to a low-temperature bath, add nitrogen protection, and wait for use.
[0023] 2. Preparation of S-(-)-1,1-diphenyl-1,2-propanediol
[0024] Other conditions in the preparation steps of S-(-)-1,1-diphenyl-1,2-propanediol are the same as in Example 1, but the reaction...
Embodiment 3
[0026] 1. Preparation of formatting reagents
[0027] Prepare a mixed solution of 170g (1.08mol) bromobenzene and 200ml butyl ether and shake well for later use. Put 31g (1.28mol) of magnesium and 200ml of butyl ether into the reaction flask, stir, and add 0.5g (0.0020mol) of iodine. Transfer the prepared bromobenzene-butyl ether mixture to a constant pressure dropping funnel, add 10-30ml of bromobenzene-butyl ether mixture dropwise into the reaction vessel, raise the temperature to 25°C, trigger the Grignard reaction, and start adding the remaining Bromobenzene-butyl ether solution, keep the reaction temperature not lower than 55°C. After the dropwise addition, keep the reaction for 4 hours, transfer the device to a low-temperature bath, add nitrogen protection, and set aside.
[0028] 2. Preparation of S-(-)-1,1-diphenyl-1,2-propanediol
[0029] Other conditions in the preparation steps of S-(-)-1,1-diphenyl-1,2-propanediol are the same as in Example 1, but the reaction c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com