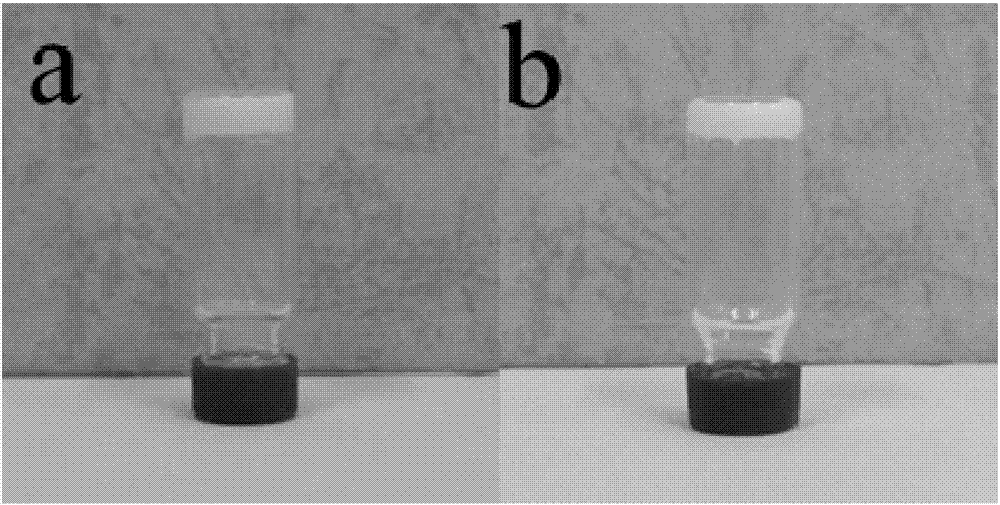
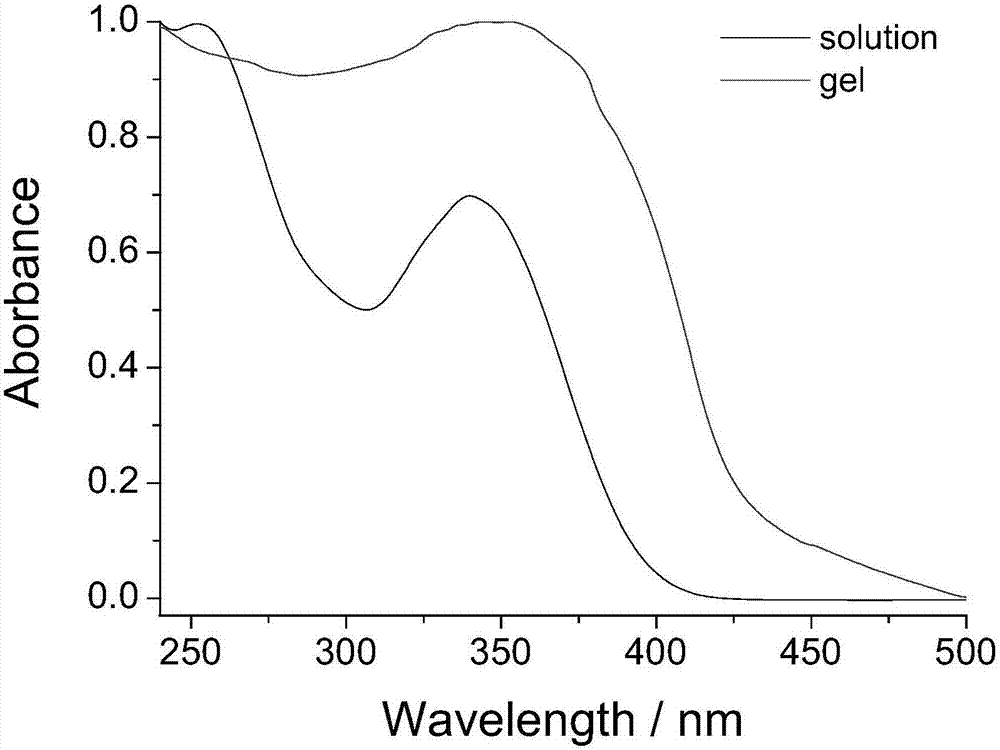
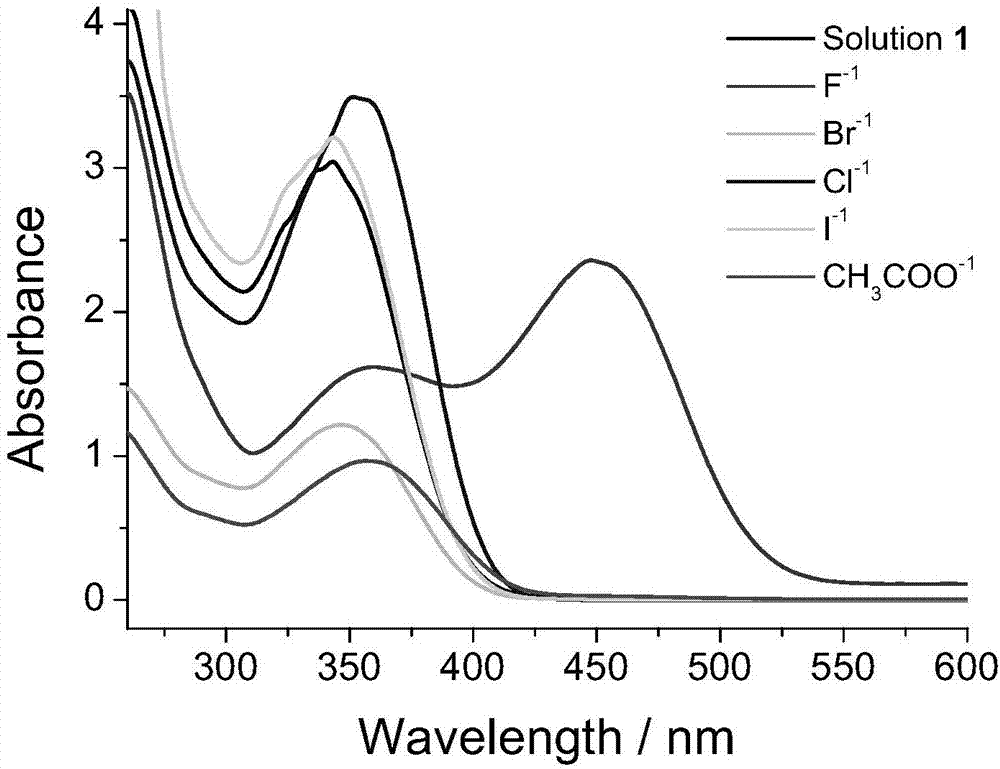
Organogel compound of 4-nitrophenylthiourea, and preparation method, gel and applications of organogel compound
A technology of nitrophenylthiourea and organogel, which is applied in the field of supramolecular chemistry and can solve the problems of few reports on mercury ion detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0035] As shown in Figure 6, the synthesis process of compound P-12 is as follows: compound p-nitrophenylisothiocyanate (0.50 g, 2.78 mmol), N-(2-aminoethyl)-3,4,5- Tris(dodecyl)benzamide (1.99 g, 2.78 mmol) was added to anhydrous tetrahydrofuran (60 mL), and stirred under reflux for 12 hours. The reaction was monitored by thin-layer chromatography, and after the reaction was completed, tetrahydrofuran was distilled off under reduced pressure to obtain a crude product. The crude product was purified by column chromatography (Methanol / CHCl 2 =1 / 30, v / v) to obtain a pale yellow solid. 1 HNMR (600 MHz, CDCl 3): 8.854 (s, 1H), 8.176 (d, 2H, J =9.0 Hz), 7.898 (s, 1H ), 7.633 (s, 2H), 7.326 (s, 1H), 6.970 (s, 2H ), 3.993 (t, 4H, J = 6.3 Hz), 3.924 (t, 4H, J = 6.3 Hz), 3.633 (s, 2H), 1.774 (m, 6H), 1.479 (m, 6H), 1.325-1.250 (m, 48H), 0.879 (t, 9H, J = 6.9 Hz), 13 CNMR (100MHz, CDCl 3 ): 169.39, 153.30, 143.97, 141.58, 127.67, 124.70, 122.20, 105.56,73.70, 69.47, 41.35, 31.94, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com