P-nitrophenoxy betulin (28) formyl ester and betulin derivatives synthesized therefrom, and preparation method and application thereof
A technology based on p-nitrophenoxy and betulin, which is applied in the direction of steroids, drug combinations, digestive system, etc., can solve the problems of poor water solubility, limited application and low bioavailability of betulin, and achieve high Bioavailability, good application prospects, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
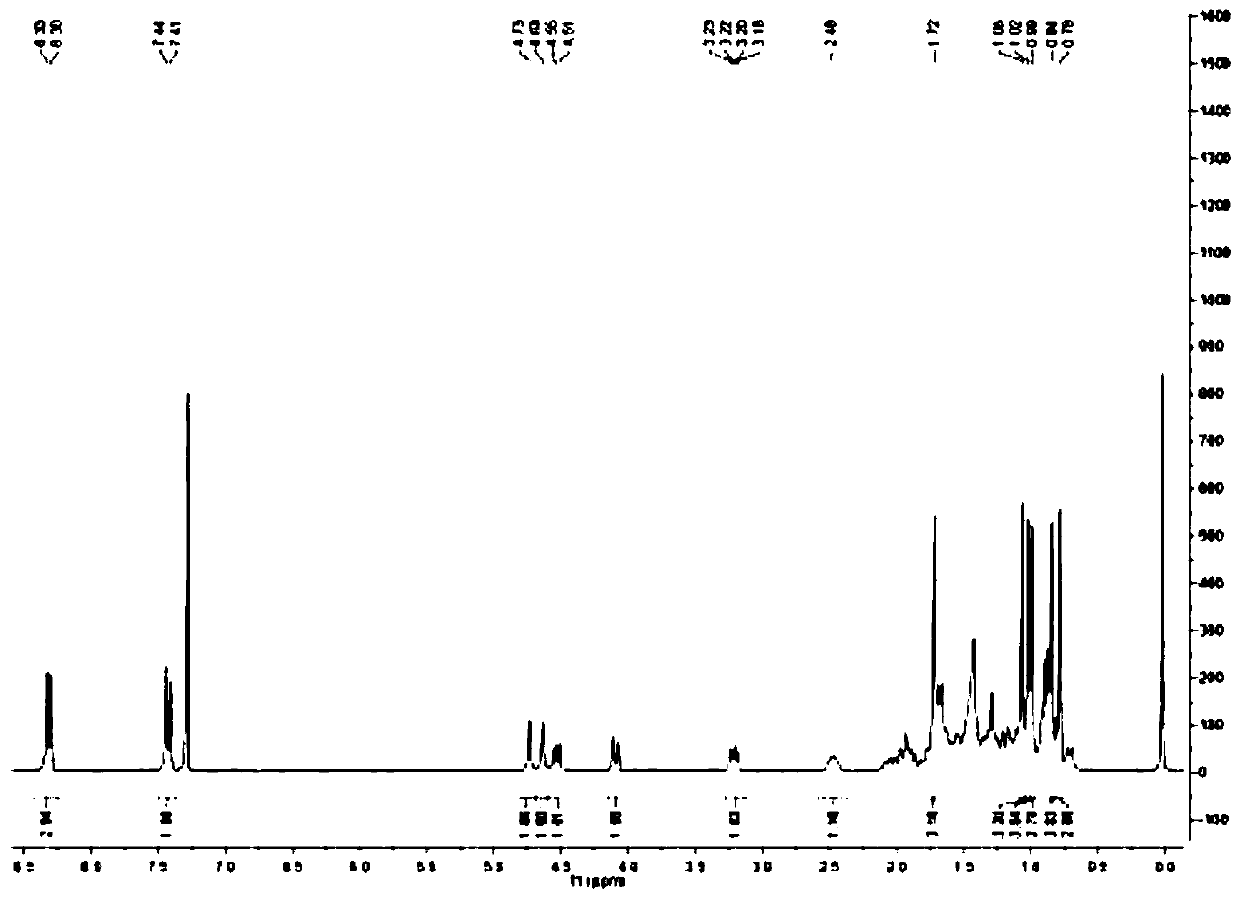
[0061] Weigh 100mg (0.23mmol) of betulin in a 25ml two-necked bottle, use 5ml of dry THF as a solvent, add 18μl (0.23mol) of pyridine as a catalyst, and add p-nitrate dissolved in THF (0.23mmol) dropwise under ice-bath conditions. 46 mg (0.23 mmol) of phenyl chloroformate, under nitrogen protection, and stirred at room temperature. TLC tracking reaction, until the betulin reaction is complete, stop stirring, evaporate the solvent under reduced pressure, silica gel column chromatography separation and purification, to obtain a white solid, which is p-nitrophenoxy betulinyl (28) formyl ester ( 77 mg, yield 55%).
[0062] The reaction equation is as follows:
[0063]
Embodiment 2
[0065] The synthetic steps of betulin derivative B-1:
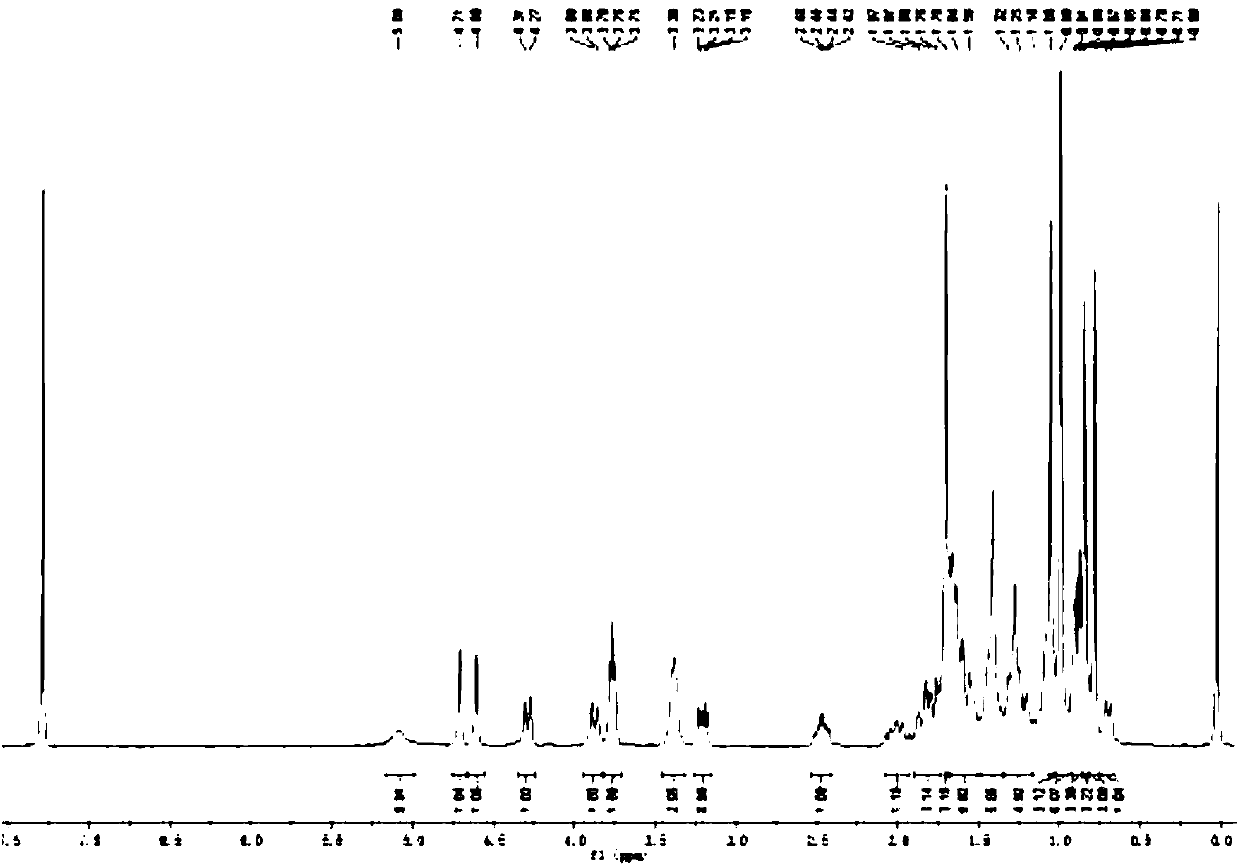
[0066] Weigh 150mg (0.08mmol) of p-nitrophenoxy betulinyl (28) formyl ester in a 25ml single-necked bottle, add 10ml of dichloromethane as a solvent, then add 5μl (0.08mmol) aminoethanol, 27mg (0.22 mmol) 4-dimethylaminopyridine (DMPA) as a catalyst, nitrogen protection, stirring at room temperature. TLC tracking, after the complete reaction of p-nitrophenoxy betulinyl (28) formyl ester, stop stirring, evaporate the solvent under reduced pressure, separate and purify by silica gel column chromatography to obtain white solid B-1 (78 mg, yield 60 %).
[0067] The reaction equation is as follows:
[0068]
Embodiment 3
[0070] The synthetic steps of betulin derivative B-2:
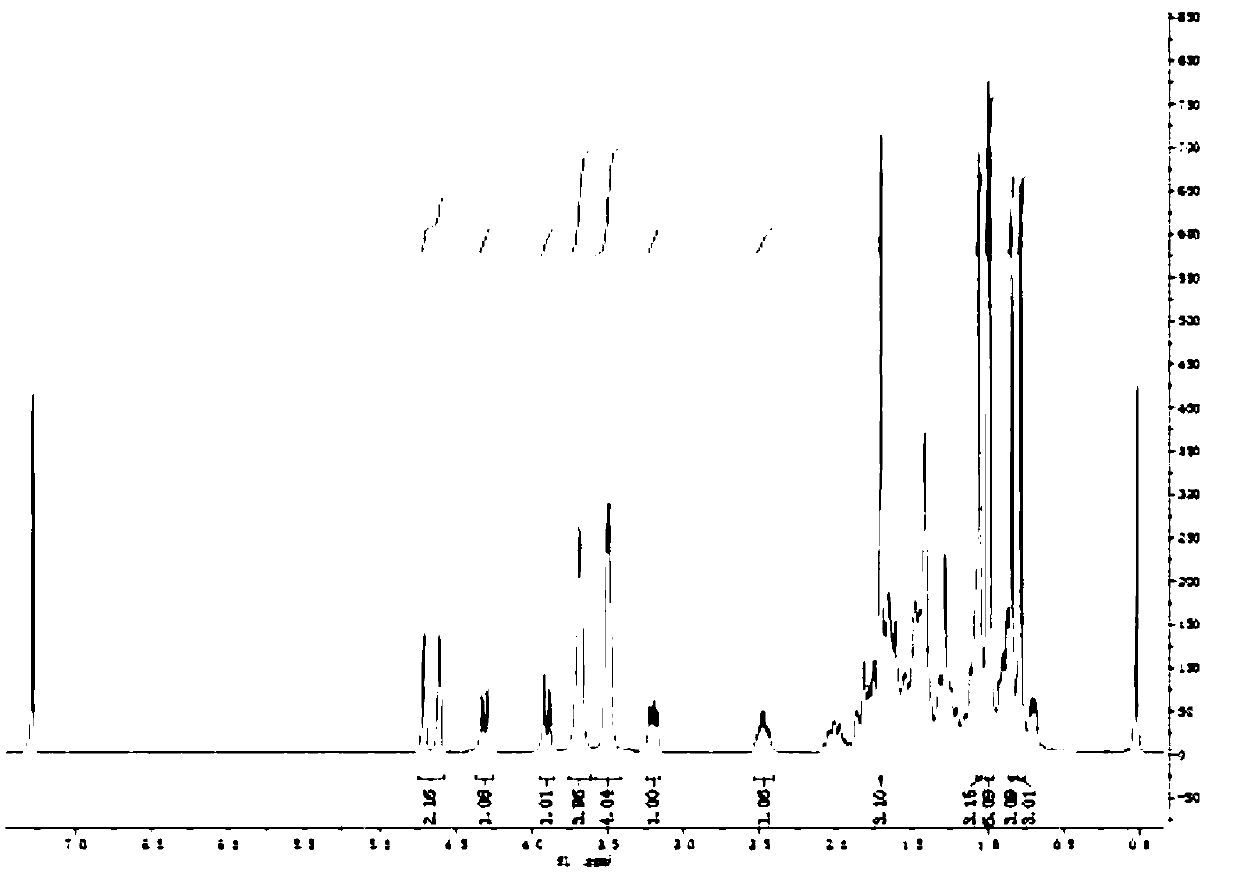
[0071] Weigh 50mg (0.07mmol) p-nitrophenoxy betulinyl (28) formyl ester in a 25ml single-necked bottle, add 10ml dichloromethane as solvent, add 8μl (0.07mmol) N-aminomorpholine, 9mg (0.07mmol) 4-dimethylaminopyridine (DMPA) as a catalyst, stirred at room temperature under nitrogen protection. TLC tracking, after the complete reaction of p-nitrophenoxy betulinyl (28) formyl ester, stop stirring, evaporate the solvent under reduced pressure, separate and purify by silica gel column chromatography to obtain white solid B-2 (26 mg, yield 60 %).
[0072] The reaction equation is as follows:
[0073]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com