Method for preparing 4-amino diphenylamine by catalytic hydrogenation
A technology of aminodiphenylamine and catalytic hydrogenation, which is applied in the field of preparation of 4-aminodiphenylamine, can solve problems such as difficulty in satisfying stable operation and production, inevitable loss of catalyst, and small proportion of catalyst cost, etc., to solve the problem of recycling, The effect of less catalyst loss and low cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0042] The alkali catalyst adopts fresh tetramethylammonium hydroxide to prepare the condensation liquid mixture after the reaction of nitrobenzene and aniline.
[0043] Concentrate 285 g of 25% tetramethylammonium hydroxide aqueous solution to 35% at 55° C. and 3000 Pa. Then add 347g of aniline, and distill off the azeotropic product at 65°C and 5500Pa to make the molar ratio of water and alkali = 5.5:1. Under this condition, add 96.6g of nitrobenzene dropwise to the reaction solution within 1h and react for another 4h During the process, the azeotrope is still continuously distilled, and the reaction conditions are controlled to be 75 ° C and 5500 Pa. After the reaction, the shrinkage mixture is 500 g.
[0044] The condensate was analyzed by high-performance liquid chromatography, and the mass fractions of each component were: aniline 64.20%, nitrobenzene 0.73%, 4-nitrosodiphenylamine 26.60%, 4-nitrodiphenylamine 4.64%, phenazine 0.19% , Azobenzene 3.63%.
Embodiment 1B
[0046] The alkali catalyst adopts recovered tetramethylammonium hydroxide, and adds 4% fresh tetramethylammonium hydroxide to prepare the condensation liquid mixture after the reaction of nitrobenzene and aniline.
[0047] 402g of the water layer recovered after a hydrogenation reaction (the mass fraction of tetramethylammonium hydroxide was measured as 17.05%, and the recovery rate was 96.2%) was concentrated to 35% at 55°C and 3000Pa, and then 7.8g was added Fresh 35% tetramethylammonium hydroxide solution. Other operating procedures are the same as in Example 1A.
[0048] The condensate was analyzed by high-performance liquid chromatography, and the mass fractions of each component were: aniline 64.21%, nitrobenzene 0.65%, 4-nitrosodiphenylamine 27.3%, 4-nitrodiphenylamine 4.15%, phenazine 0.21% , Azobenzene 3.48%. Tetramethylammonium hydroxide has no obvious effect on the condensation reaction after being recovered and used mechanically.
Embodiment 2A
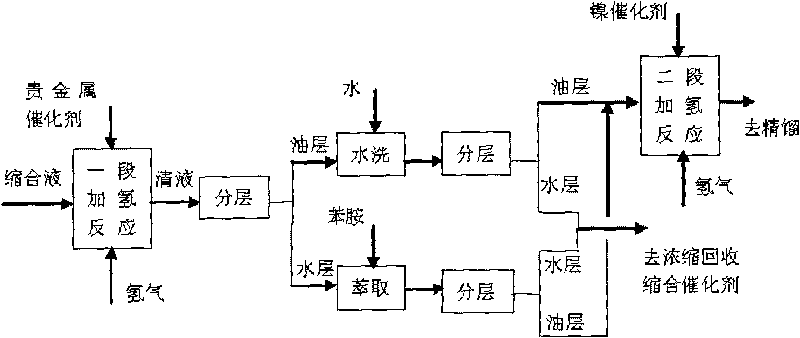
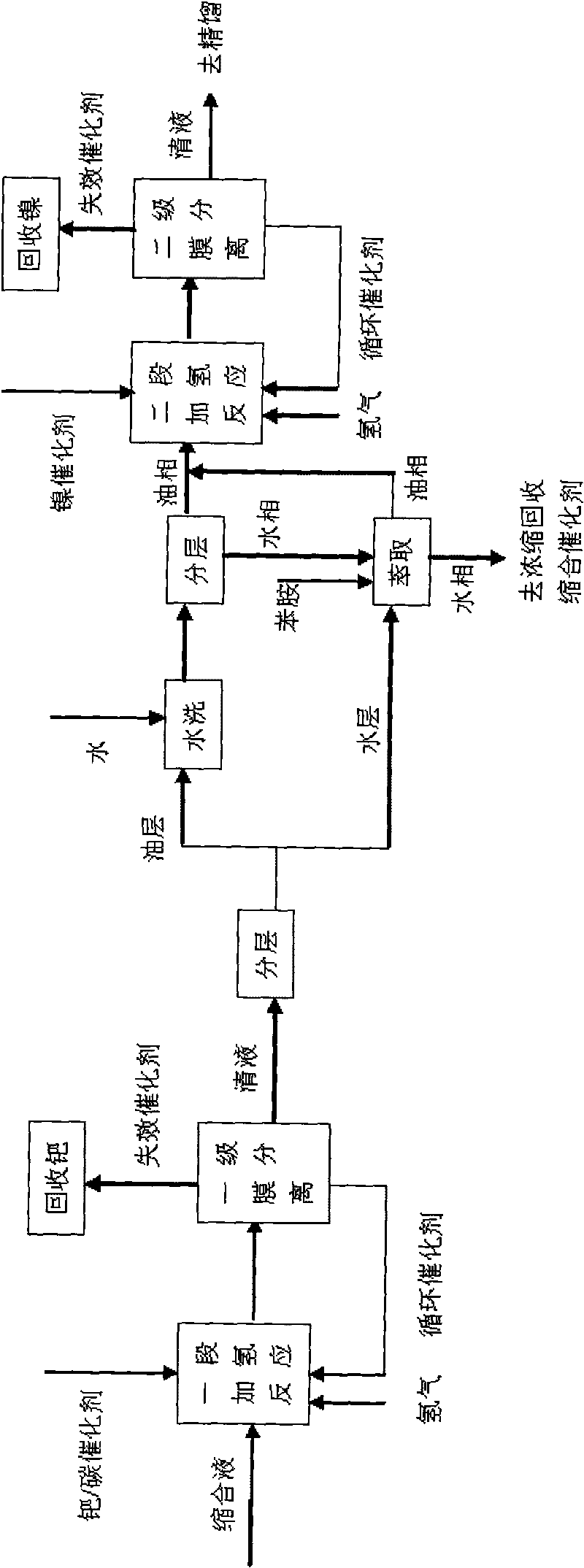
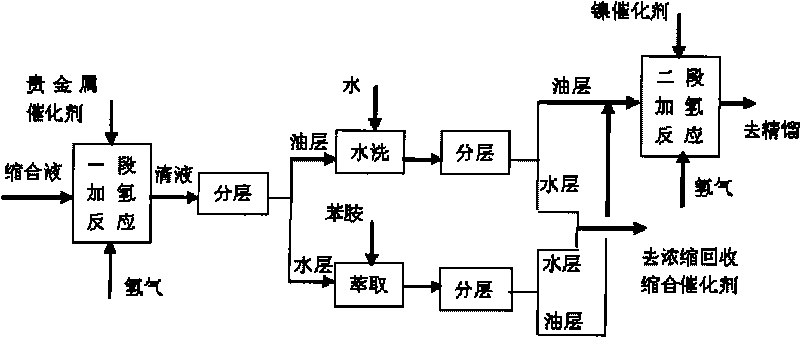
[0050] Attached figure 1 In the two-stage catalytic hydrogenation process shown, the first-stage hydrogenation catalyst is a commercially available 5% palladium / carbon catalyst, and the second-stage hydrogenation catalyst is a self-made ordinary skeleton nickel catalyst made of aluminum-nickel alloy powder.
[0051] The condensation solution 500g of embodiment 1A is put into one-stage hydrogenation autoclave, add 150g water and 1.5g 5% palladium / carbon catalyst simultaneously, replace 4 times with nitrogen and hydrogen respectively, feed hydrogen then, control reactor pressure is 3.5MPa, the temperature is 70°C, and the reaction is over when no hydrogen is absorbed; after cooling down to room temperature, the reaction liquid is released through the filter valve, and the catalyst is left in the kettle to continue to apply; To neutrality, the washing water and the separated water layer are combined and extracted with 150g aniline, and the extracted water layer is applied mechani...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com