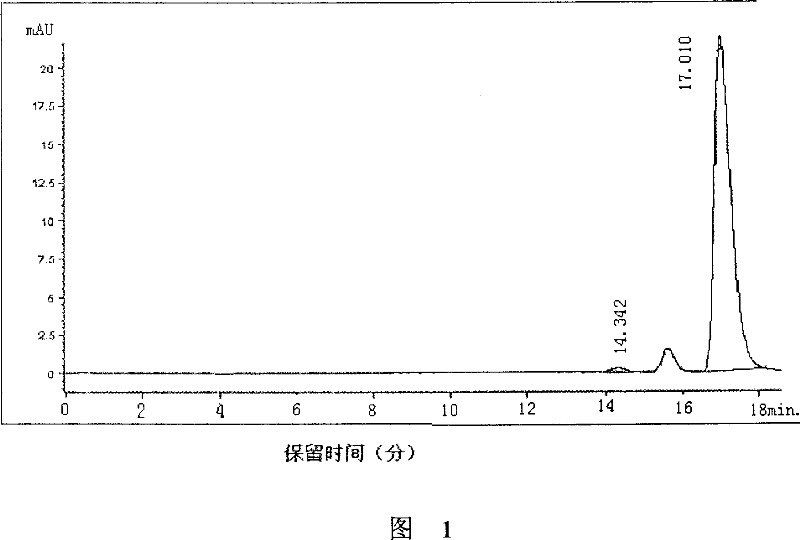
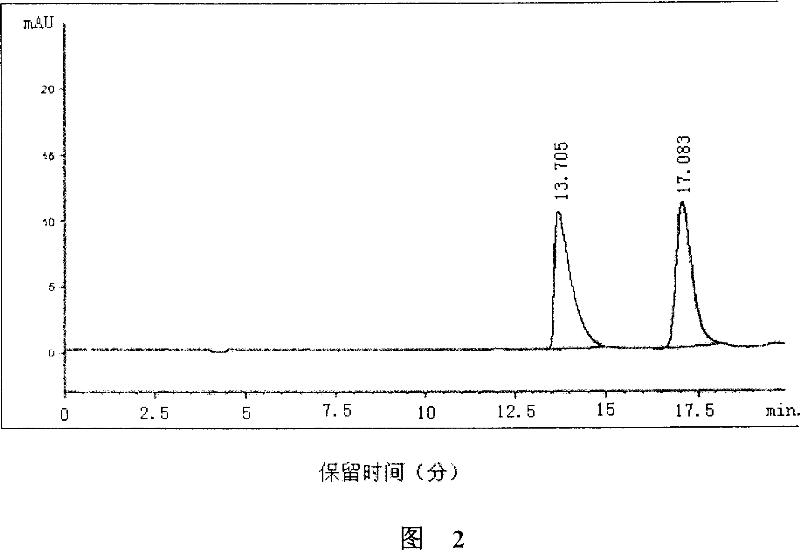
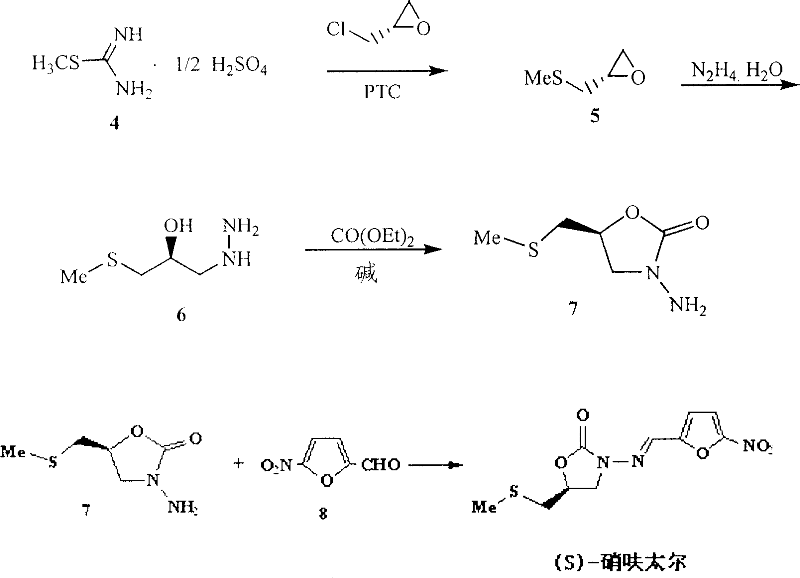
(S)-nifuratel, preparation method and application thereof
A nifuratel, reaction technology, applied in the direction of pharmaceutical formula, medical preparations containing active ingredients, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0049] Preparation of Example 1 Intermediate 4
[0050] Intermediate 4 was prepared according to the following reaction:
[0051]
[0052] In a 2000mL reaction flask equipped with a high-efficiency reflux condenser and a dropping funnel, add 250g (3.125mol) of thiourea and 110mL of water, stir to partially dissolve the thiourea, stir for 10min under ice water cooling, remove the ice water, and add dropwise Dimethyl sulfate 15mL, the reaction spontaneously started under stirring, and exothermic, very violent at the beginning, after the reaction was stable, then slowly add the remaining dimethyl sulfate 155mL in the reaction bottle (about 1.5h), always Maintain a slightly boiling state, after the addition, continue to reflux for 1 hour, let stand overnight, add 260 mL of 95% ethanol, stir for a while, cool, filter, wash twice with 95% ethanol, and dry to obtain 4364 g of the product, with a yield of 84%, mp. 236°C (dec).
preparation Embodiment 2
[0053] Preparation Example 2. Preparation of Intermediate 5
[0054]
[0055] Ingredients:
[0056]
Reagent name
molecular weight
weight
(g)
moles
(mole)
level
Manufacturer
Remark
4
139
139g
1.0
self made
(S)-Epichlorohydrin
92.5
78.5mL
1.0
A.R.
Aldrich
Catalyst tetra-n-butyl
6.4g
TBAB
500ml
A.R.
beijing chemical reagent factory
30%NaOH
500mL
A.R.
beijing chemical reagent factory
self-provided
[0057] operate:
[0058] Add 4139g (1.0mol), (S)-epichlorohydrin 78.5mL (92.6g, 1.0mol), benzene 500mL, 30% sodium hydroxide 500mL and catalyst tetra-n-butylammonium bromide 6.4g in 2000mL reaction flask , reacted at room temperature for 6 hours under high-efficiency stirring, separated the ...
preparation Embodiment 3
[0061] Preparation Example 3. Preparation of Intermediate 6
[0062]
[0063]
Reagent name
molecular weight
weight
(g)
moles
(mole)
level
Manufacturer
5
104
88.5g
0.85
self made
Hydrazine hydrate
200mL
85%
Beijing Chemical Plant
[0064] operate:
[0065] Add 200mL of hydrazine hydrate into the reaction flask, heat to 90°C, add 588.5g (0.85mol) dropwise with stirring, and control the internal temperature at about 90°C, complete the addition in about 0.5h, and continue the reaction at 90°C for 1h. Excess hydrazine and water were removed by rotary evaporation to obtain a viscous colorless liquid. It can be directly used in the next reaction without purification, and the yield is quantitative.
[0066] If the obtained crude product is purified by vacuum distillation, a colorless transparent viscous pure product 6 is obtained, with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com