Preparation and application of N-heterocyclic carbene gold porous organic polymer
A nitrogen heterocyclic carbene and polymer technology is applied in the field of preparation and application of porous organic polymers, which can solve the problems of unmaintained activity, long synthesis cycle, long preparation route, etc., and achieves short synthesis cycle, easy separation, and preparation technology. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
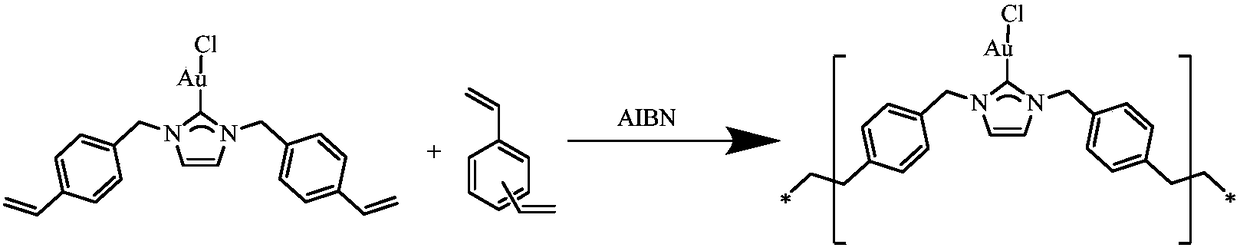
[0018] Weigh 0.27g 1,3-bis(p-vinylbenzyl) imidazocarbene gold chloride (I) and 0.65g divinylbenzene (DVB) and dissolve in 27mg azobisisobutyronitrile (AIBN), and then Add 5mL of DMF, pre-polymerize and stir for 30min at room temperature, then react at 80°C for 24h, after the reaction, wash the filtrate three times with DMF and acetone, and then vacuum-dry to obtain 0.83g of azacyclic carbene gold porous organic polymers.
[0019] See attached figure 1 , the above product was characterized by scanning electron microscopy, and the obtained polymer was a particle packing material.
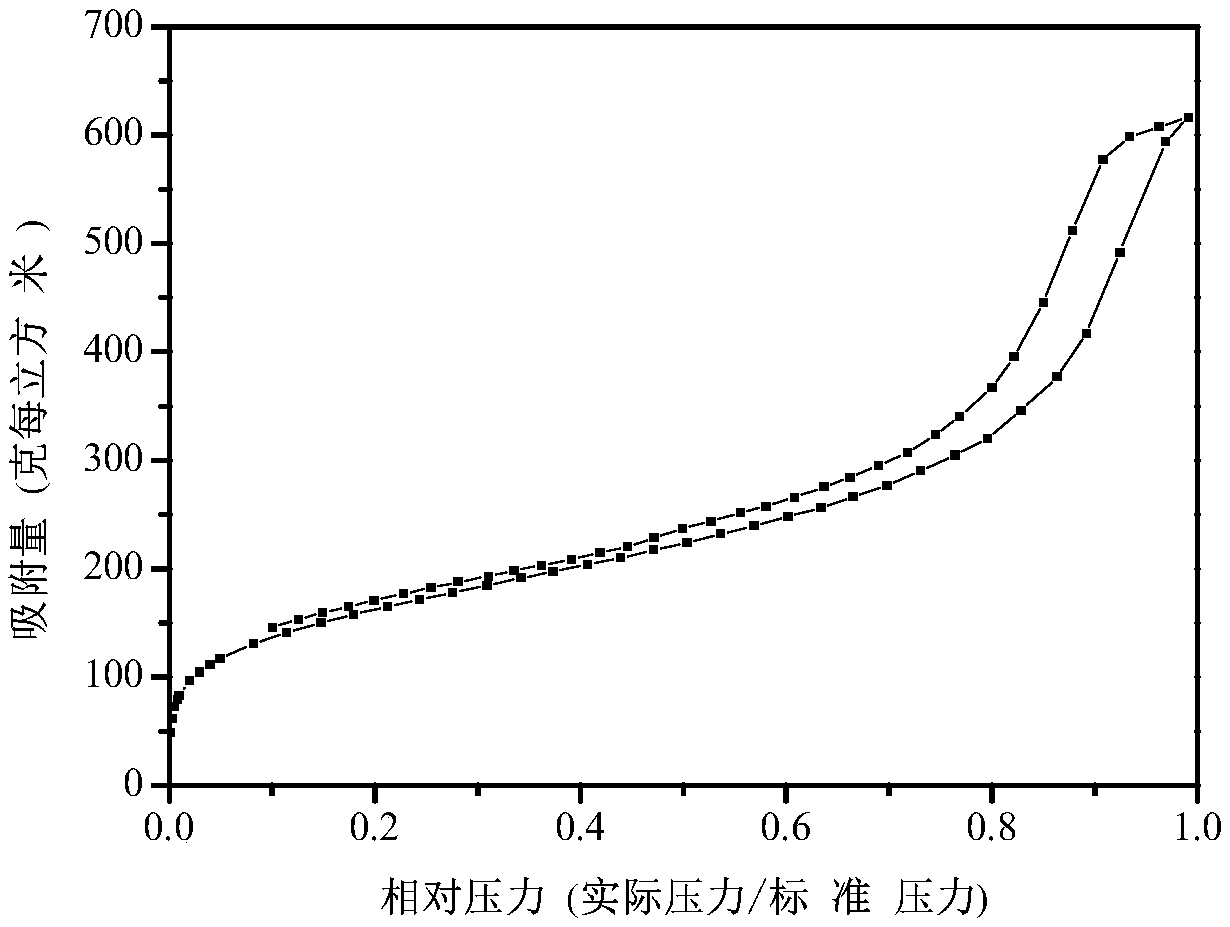
[0020] See attached figure 2 , the above product was measured by nitrogen adsorption-desorption isothermal characterization, and its specific surface area was 572m 2 g -1 , the pore volume is 0.54cm 3 g -1 .
Embodiment 2
[0022] Weigh 0.27g 1,3-bis(p-vinylbenzyl) imidazocarbene gold chloride (I) and 0.33g divinylbenzene (DVB) and dissolve in 27mg azobisisobutyronitrile (AIBN), and then Add 10mL of DMF, pre-polymerize and stir for 30min at room temperature, and then react at 80°C for 20h. After the reaction is completed, the filtrate is washed with DMF and acetone three times and then vacuum-dried to obtain 0.55g of the product. organic polymers.
Embodiment 3
[0024] Weigh 0.27g 1,3-bis(p-vinylbenzyl) imidazocarbene gold chloride (I) and 1.30g divinylbenzene (DVB) and dissolve in 50mg azobisisobutyronitrile (AIBN), and then Add 15mL of DMF, pre-polymerize and stir for 30min at room temperature, and then react at 80°C for 24h. After the reaction is completed, the filtrate is washed with DMF and acetone three times and then vacuum-dried to obtain 1.46g of the product. organic polymers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com