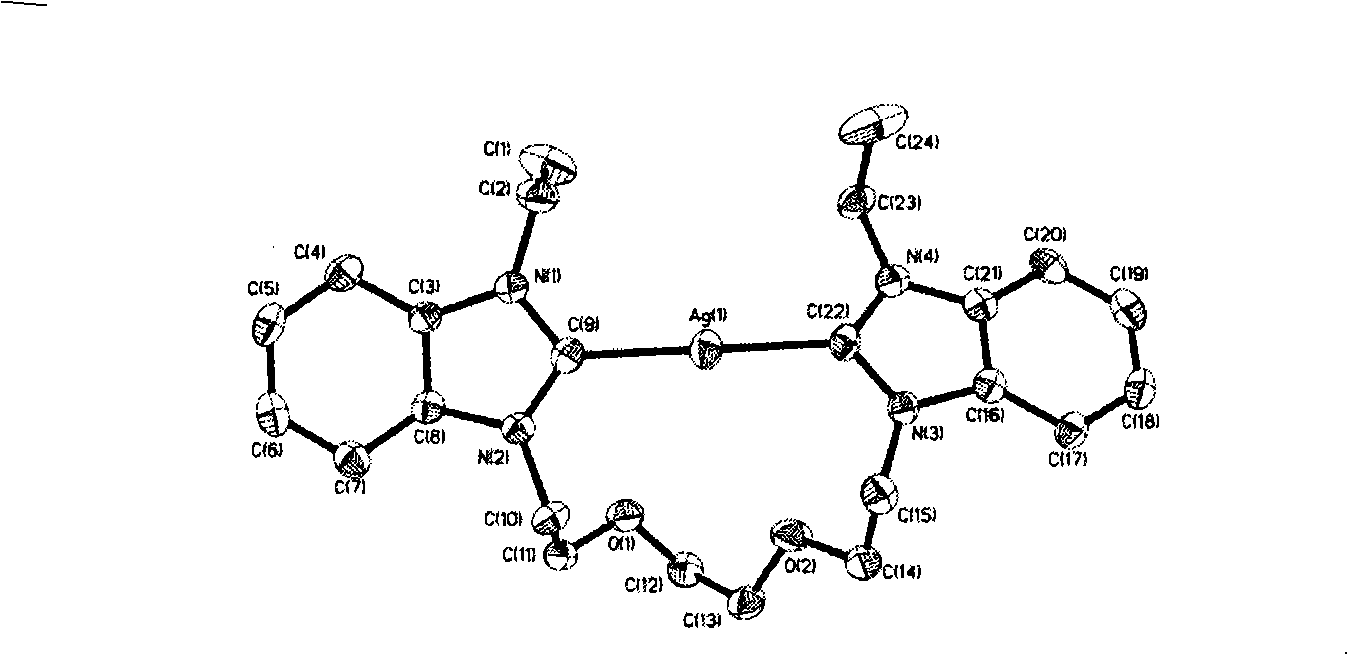
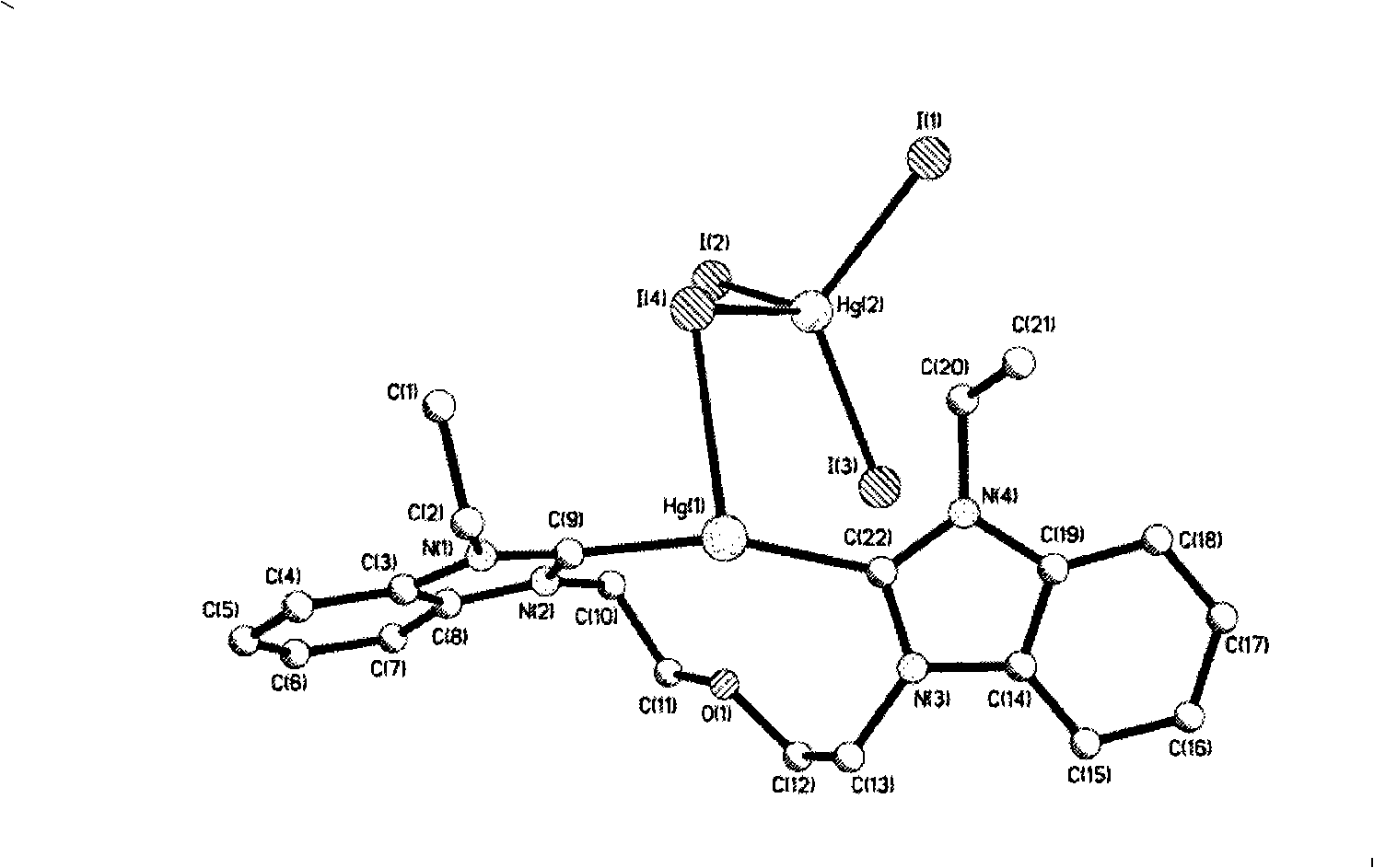
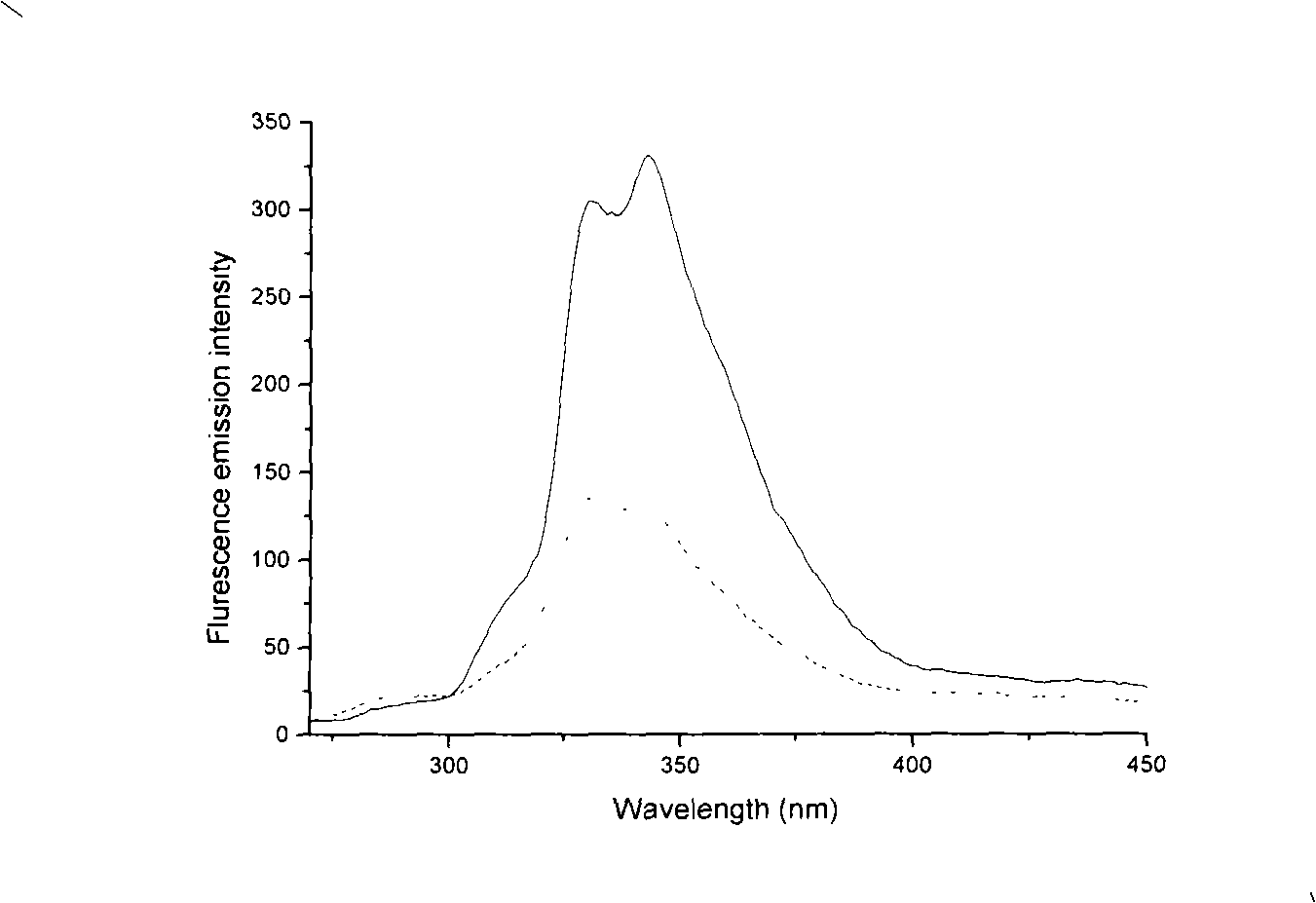
N-heterocyclic dicarbene metal complex connected with ether chain, method for preparing same and use
A nitrogen heterocyclic carbene and metal complex technology, applied in the direction of mercury organic compounds, copper organic compounds, silver organic compounds, etc., can solve problems such as metal complex research only, achieve adjustable structure, obvious fluorescence photosensitive effect, Prepare concise effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0053] 1,2-bis(2'-iodoethoxy)ethane (1.500 g, 4.0 mmol) and 1-ethylbenzimidazole (1.304 g, 8.9 mmol) were added to 1,4-dioxane, After stirring at 90°C for 3 days, a white precipitate precipitated out, which was filtered and washed with ether to obtain a white powder of 1,2-bis[2'-(3-ethylbenzimidazole)ethoxy]ethane iodide. Yield: 2.060 g (76.6%), Mp: 192-194°C. 1 H NMR (300MHz, DMSO-d 6 ): δ1.62 (t, J=5.5, 6H, CH 3 ), 3.36(t, J=4.2, 4H, CH 2 ), 3.78(t, J=4.2, 4H, CH 2 ), 4.72(t, J=4.2, 4H, CH 2 ), 4.86 (q, J=5.5, 4H, CH 2 ), 7.68 (m, 4H, PhH), 7.90 (d, J=6.3, 2H, PhH), 7.97 (d, J=6.3, 2H, PhH), 11.50 (s, 2H, 2-benzimiH) (benzimi= benzimidazole).
[0054] The experimental process is shown in the figure below:
[0055]
preparation example 2
[0057] 1,2-bis(2'-iodoethoxy)ethane (1.500 g, 4.1 mmol) and 1-pyridylbenzimidazole (2.044 g, 8.9 mmol) in 1,4-dioxane, Stirring at 90°C for 3 days, a white precipitate precipitated, filtered and washed with ether to obtain 1,2-bis[2'-(3-(2"-pyridylmethyl)benzimidazole)ethoxy]ethane iodide Compound as a white powder. Yield: 2.540 g (75.6%), Mp: 156-158°C. 1 H NMR (300MHz, DMSO-d 6 ): δ3.76 (t, J=4.2, 4H, CH 2 ), 4.74(t, J=4.2, 4H, CH 2 ), 4.85(t, J=5.5, 4H, CH 2 ), 5.93 (s, 4H, CH 2 ), 7.39(m, 2H, PhH), 7.66(m, 6H, PhH or PyH), 7.93(m, 4H, PhH or PyH), 8.18(m, 2H, PyH), 8.46(d, 2H, PyH) , 9.87 (s, 2H, 2-benzimiH) (benzimi=benzimidazole).
[0058] The experimental process is shown in the figure below:
[0059]
preparation example 3
[0061] Bis(2-iodoethyl)ether (5.000g, 0.015mol) and 1-ethylbenzimidazole (4.930g, 0.034mol) in 1,4-dioxane were stirred at 90°C for 3 days, A white precipitate precipitated, was filtered and washed with ether to obtain a white powder of bis[2-(3-ethyl)benzimidazole]ether iodide. Yield: 7.460 g (78.7%), Mp: 172-174°C. 1 H NMR (300MHz, DMSO-d 6 ): δ1.64 (t, J=5.5, 6H, CH 3 ), 3.41 (q, J = 4.2, 4H, CH 2 ), 3.71(t, J=4.2, 4H, CH 2 ), 4.77(t, J=4.2, 4H, CH 2 ), 7.70 (m, 4H, PhH), 7.92 (d, J=6.4, 2H, PhH), 7.94 (d, J=6.4, 2H, PhH), 11.23 (s, 2H, 2-benzimiH) (benzimi= benzimidazole).
[0062] The experimental process is shown in the figure below:
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com