Preparation method of acid hydrolytic impurity in linezolid
A technology of linezolid and acidic hydrolysis, applied in the field of medicine, can solve problems such as potential safety hazards, achieve the effects of convenient operation, simple preparation conditions, and avoid side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
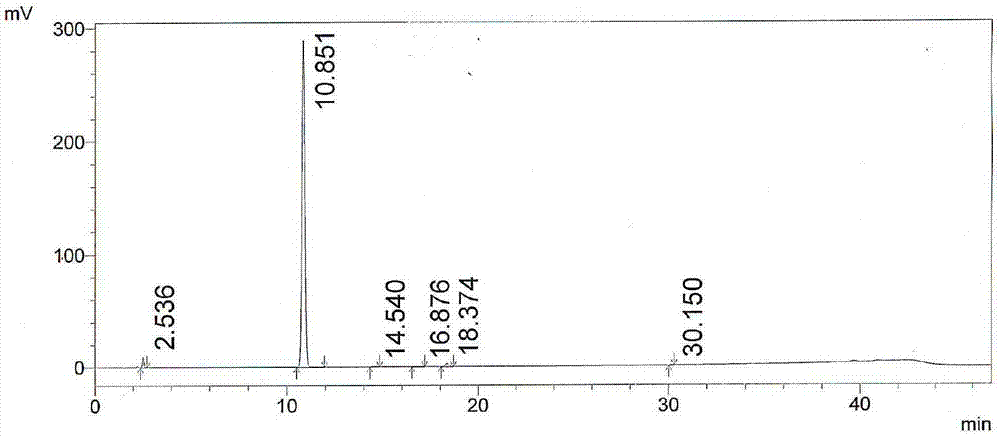
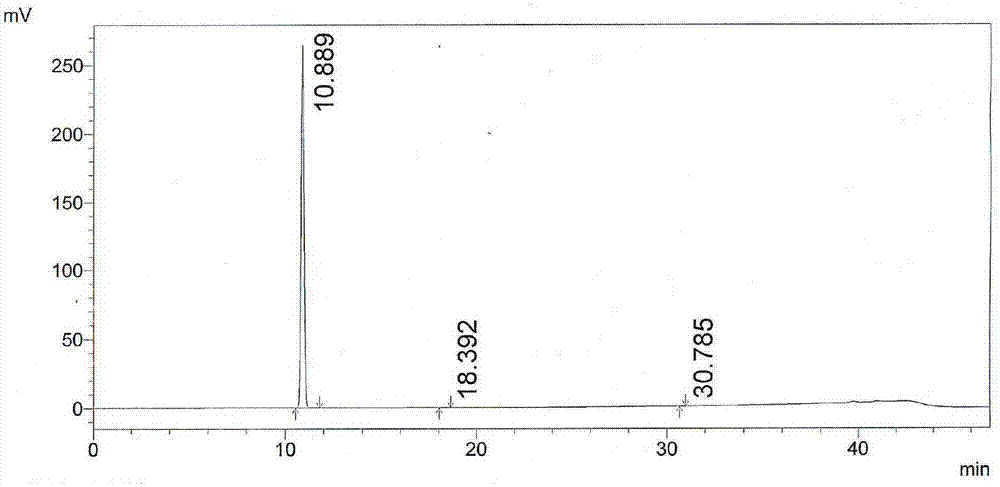
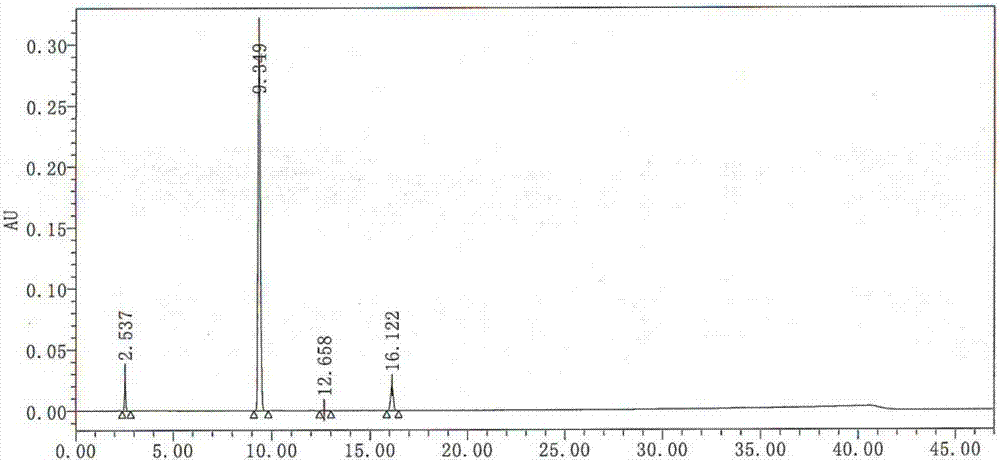
Image
Examples
Embodiment 1
[0039] The preparation method of the acidic hydrolysis impurity of linezolid in this embodiment comprises the following steps:
[0040] (1) Hydrolysis: In a 250ml round-bottomed flask, take 10g of linezolid shown in formula II, add 20ml of 6mol / L hydrochloric acid dropwise under 40g of absolute ethanol, acid hydrolyze at 70°C for 20h, remove the acetyl group, and cool down to 20 DEG C, obtain the hydrochloride of formula I;
[0041] (2) Distillation: steaming dehydrated alcohol described in step (1);
[0042] (3) Alkalization and extraction and purification: the pH of the solution is adjusted to 7 with a mass percent concentration of 5% sodium hydroxide solution, filtered to obtain a red filtrate and filter cake; the filtrate is extracted with n-butanol 30ml × 2 to obtain n-butanol Alcohol extract 1; the filter cake was stirred with dichloromethane for 30 minutes, filtered, washed with dichloromethane, dissolved in 20ml of water, and added with a mass percent concentration of...
Embodiment 2
[0047] The preparation method of the acidic hydrolysis impurity of linezolid in this embodiment comprises the following steps:
[0048] (1) Hydrolysis: In a 250ml round-bottomed flask, take 10g of linezolid shown in formula II, add 20ml of 4mol / L hydrochloric acid dropwise under 50g of anhydrous methanol, and heat under reflux at 80°C for 16 hours to remove the acetyl group. Cool down to 22°C to obtain the hydrochloride of formula I;
[0049] (2) Distillation: steam the anhydrous methanol described in step (1);
[0050] (3) Alkalization and extraction and purification: the pH of the solution is adjusted to 8 with a mass percent concentration of 5% sodium hydroxide solution, filtered to obtain a red filtrate and filter cake; the filtrate is extracted with n-butanol 30ml × 2 to obtain n-butanol Alcohol extract 1; the filter cake was stirred with chloroform for 30 minutes, filtered, washed with chloroform, the insoluble matter was dissolved in 20ml of water, and the pH of the so...
Embodiment 3
[0055] The preparation method of the acidic hydrolysis impurity of linezolid in this embodiment comprises the following steps:
[0056] (1) Hydrolysis: In a 250ml round-bottomed flask, take 10g of linezolid, add 20ml of 7.5mol / L hydrochloric acid dropwise under 80g of anhydrous methanol, perform acidic hydrolysis at 90°C for 10h, remove acetyl groups, cool down to 20°C, Obtain the hydrochloride of formula I;
[0057] (2) Distillation: steam the anhydrous methanol described in step (1);
[0058] (3) Alkalization and extraction and purification: it is 10 to adjust the pH of the solution with a mass percent concentration of 5% potassium hydroxide solution, and filter to obtain a red filtrate and filter cake; the filtrate is extracted with n-butanol 30ml×2 to obtain n-butanol Alcohol extract 1; the filter cake was stirred with dichloromethane for 30 minutes, filtered, washed with dichloromethane, dissolved in 20ml of water, and 5% potassium hydroxide solution was added to adjust ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com