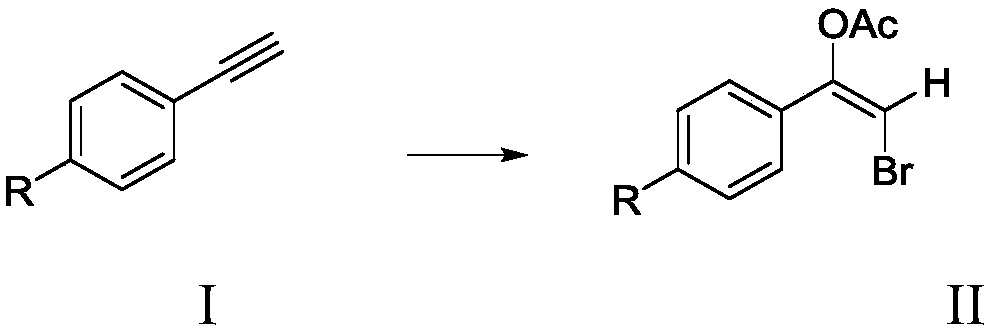
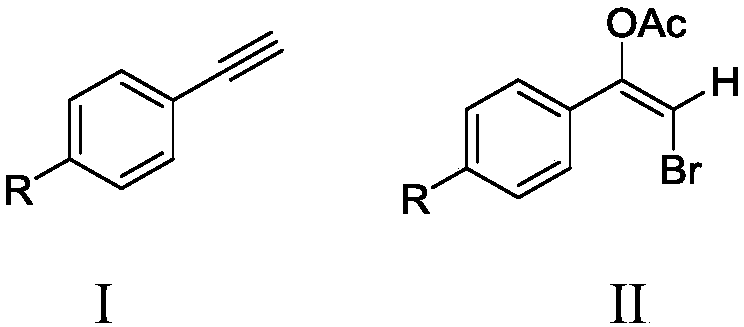
Method for synthesizing alkyne bromoacetate compounds
A technology of alkyne bromoacetate and a synthesis method, which is applied in the field of organic compound synthesis, can solve the problems of narrow substrate expansion, poor chemical selectivity, harsh reaction conditions and the like, achieves environmental friendliness, simplified reaction process and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 2mmol (0.2043g) of phenylacetylene and 1.2mmol (0.1428g) of potassium bromide into a 50mL three-necked flask, then add 20mL of acetic acid as a solvent, and then add 0.7g of bromate-intercalated zinc-aluminum hydrotalcite ZnAl-BrO 3 - - LDHs, stirred magnetically at 40°C for 4 hours. After the reaction is over, use a centrifuge at 6000r / min to centrifuge to remove the zinc-aluminum hydrotalcite solid, place the resulting liquid in a separatory funnel, add dichloromethane and deionized water, and extract the organic matter obtained from the reaction into the dichloromethane phase to obtain Column chromatography silica gel was added to the solution, the solvent was distilled off under reduced pressure, and the remaining mixture was separated by column chromatography, using a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 15:1 as the eluent, and the eluted fraction containing the product was collected. solution, and the eluent was evaporated...
Embodiment 2
[0031] Add 2mmol (0.2043g) of phenylacetylene and 1.0mmol (0.1190g) of potassium bromide into a 50mL three-necked flask, then add 16mL of acetic acid as a solvent, and then add 0.6g of bromate-intercalated zinc-aluminum hydrotalcite ZnAl-BrO3 - - LDHs, stirred magnetically at 60°C for 6 hours. After the reaction is over, use a centrifuge at 6000r / min to centrifuge to remove the zinc-aluminum hydrotalcite solid, place the resulting liquid in a separatory funnel, add dichloromethane and deionized water, and extract the organic matter obtained from the reaction into the dichloromethane phase to obtain Column chromatography silica gel was added to the solution, the solvent was distilled off under reduced pressure, and the remaining mixture was separated by column chromatography, using a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 15:1 as the eluent, and the eluted fraction containing the product was collected. solution, and the eluent was evaporated t...
Embodiment 3
[0034] Add 2mmol (0.2043g) of phenylacetylene and 1.6mmol (0.1904g) of potassium bromide into a 50mL three-neck flask, then add 24mL of acetic acid as a solvent, and then add 1.4g of bromate-intercalated zinc-aluminum hydrotalcite ZnAl-BrO 3- - LDHs, stirred magnetically at 30°C for 1 hour. After the reaction is over, use a centrifuge at 6000r / min to centrifuge to remove the zinc-aluminum hydrotalcite solid, place the resulting liquid in a separatory funnel, add dichloromethane and deionized water, and extract the organic matter obtained from the reaction into the dichloromethane phase to obtain Column chromatography silica gel was added to the solution, the solvent was distilled off under reduced pressure, and the remaining mixture was separated by column chromatography, using a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 15:1 as the eluent, and the eluted fraction containing the product was collected. solution, and the eluent was evaporated to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com