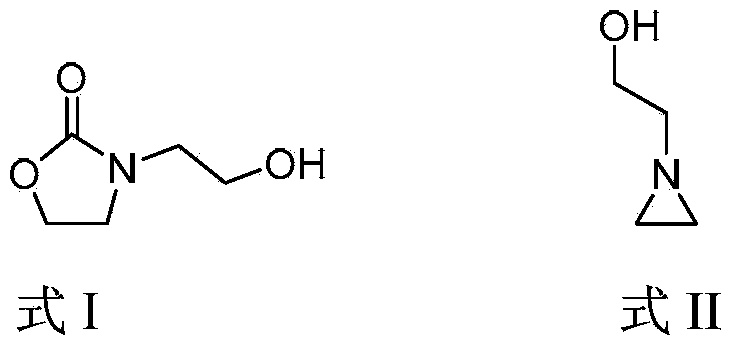
Method for preparing 3-(2-hydroxyethyl)-2-oxazolidone
A technology of oxazolidinone and hydroxyethyl, which is applied in the field of catalytic synthesis of fine chemical products, can solve the problems of many research results, and achieve the effects of high activity, stable properties, and easy separation and recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Measure 0.046g (0.2mmol) of catalyst 2,2',2"-terpyridine shown in formula III-1, 0.174g (2mmol) of compound 1-(2-hydroxyethyl) ethyleneimine shown in formula II and Add 6mL of organic solvent ethanol into a 25-mL stainless steel autoclave. After sealing the autoclave, fill it with carbon dioxide with a pressure of 30atm at 25°C. Stir and heat to 90°C. Keep warm for cyclization reaction for 20h. Then cool To room temperature, release gas.Reaction result: the yield of 3-(2-hydroxyethyl)-2-oxazolidinone is 58%.
[0033]
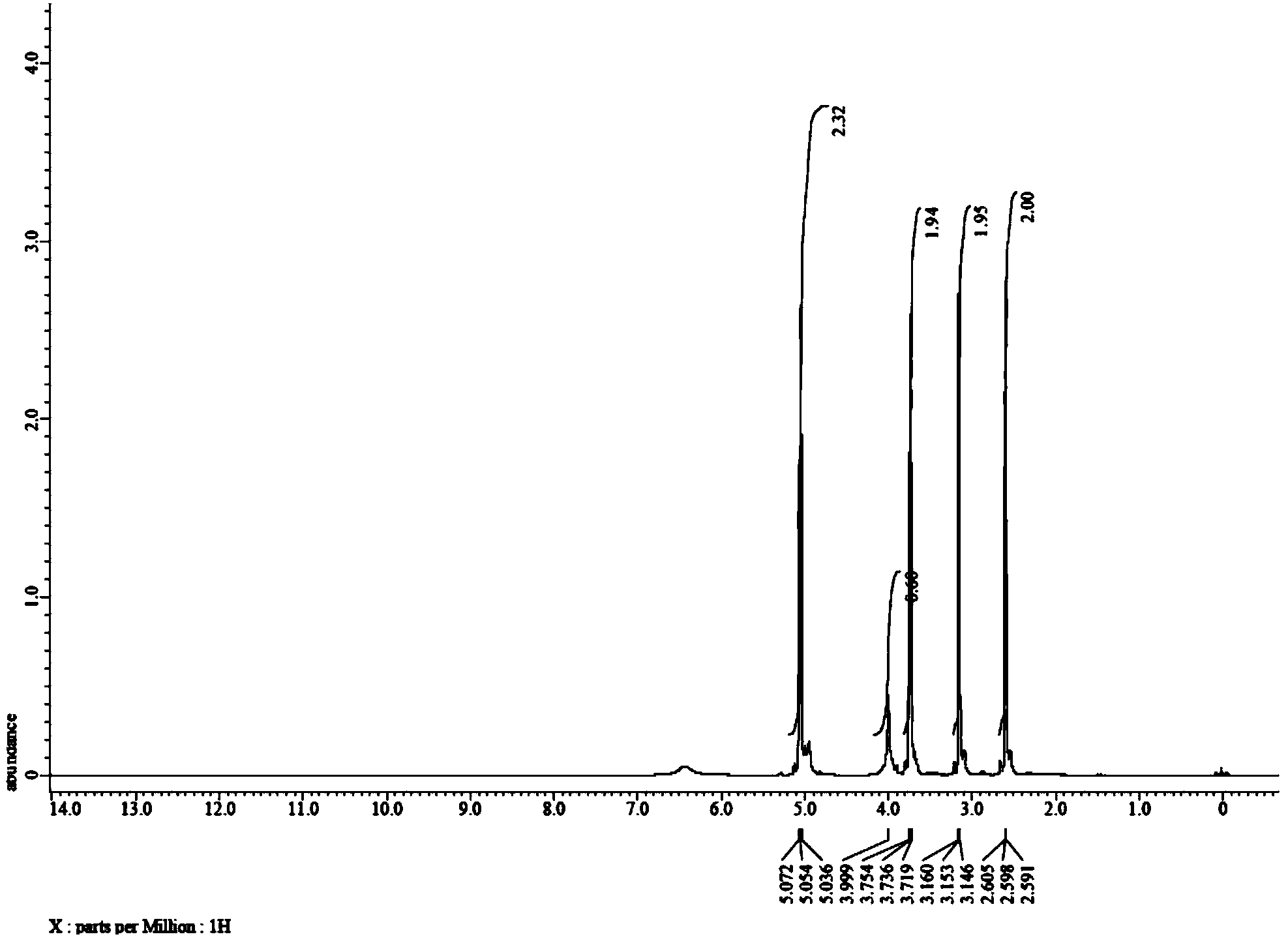
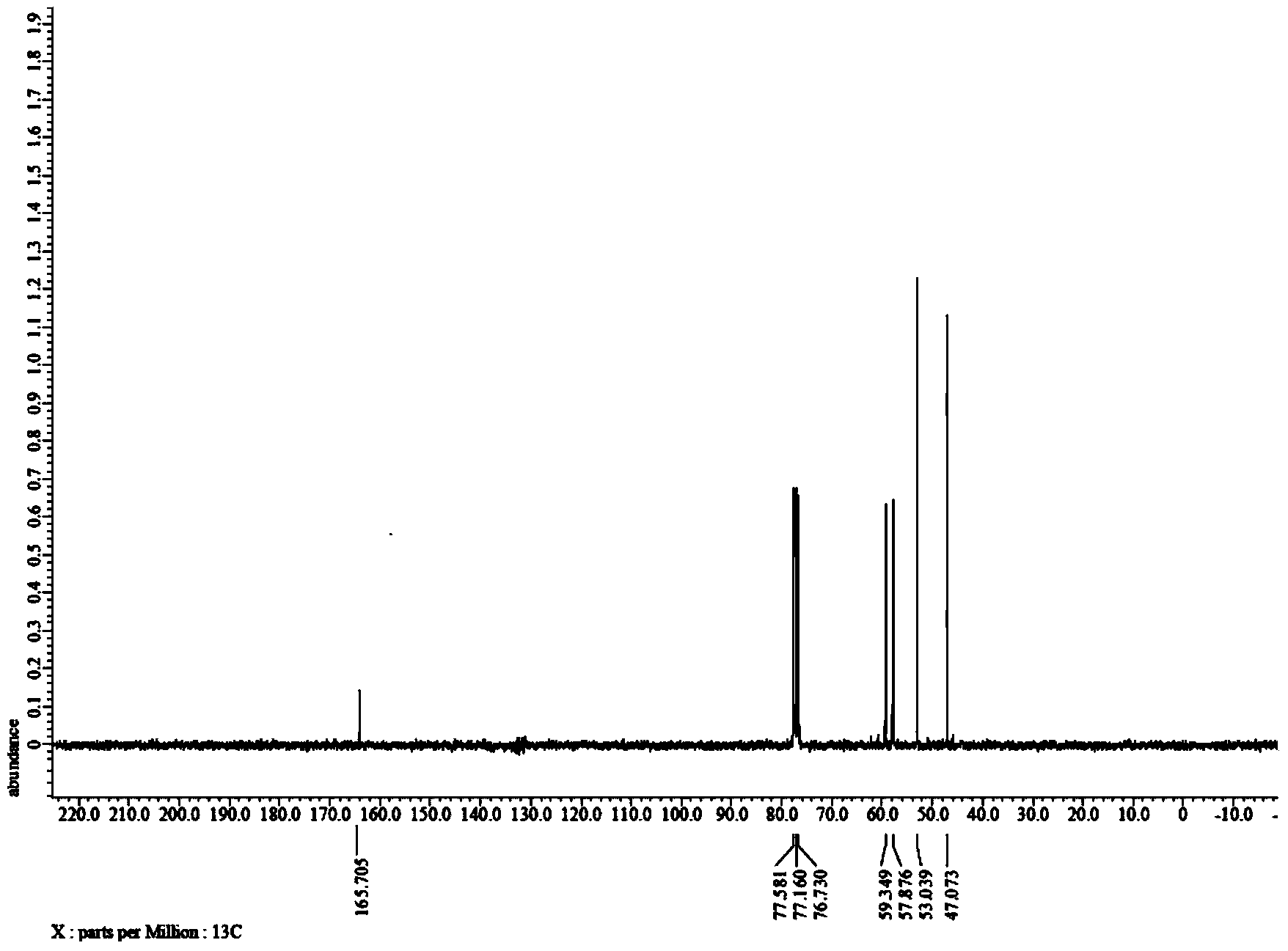
[0034] The proton nuclear magnetic spectrum of the product obtained in this embodiment is as figure 1 As shown, the carbon spectrum as figure 2 As can be seen from the figure, the product has a correct structure and is 3-(2-hydroxyethyl)-2-oxazolidinone shown in formula I.
Embodiment 2
[0036] Measure 0.046g (0.2mmol) of 2,2',2"-terpyridine, 0.174g (2mmol) of 1-(2-hydroxyethyl) ethyleneimine and 6mL methanol into a 25-mL stainless steel autoclave In. After sealing the reactor, fill it with 30atm carbon dioxide at 25°C. Stir and heat to 110°C. Keep warm for cyclization reaction for 20h. Then cool to room temperature and release gas. Reaction result: 3-(2-hydroxyethyl The yield of -2-oxazolidinone was 70%.
[0037] The NMR results of the product obtained in this embodiment are exactly the same as in Example 1, and will not be repeated here.
Embodiment 3
[0039] Measure 0.046g (0.2mmol) of 2,2',2"-terpyridine, 0.174g (2mmol) of 1-(2-hydroxyethyl) ethyleneimine and 6mL methanol into a 25-mL stainless steel autoclave In. After sealing the reactor, fill it with 30atm carbon dioxide at 25°C. Stir and heat to 130°C. Keep warm for cyclization reaction for 20h. Then cool to room temperature and release gas. Reaction result: 3-(2-hydroxyethyl The yield of -2-oxazolidinone was 60%.
[0040] The NMR results of the product obtained in this embodiment are exactly the same as in Example 1, and will not be repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com