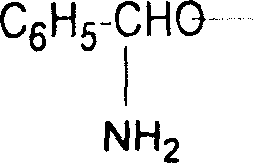Prepn process of chiral 4-substituent-2-oxazolidone
An oxazolidinone and chiral technology, which is applied in the field of preparation of chiral 4-substituted-2-oxazolidinone, achieves the effects of reducing the amount of solvent, shortening the time and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
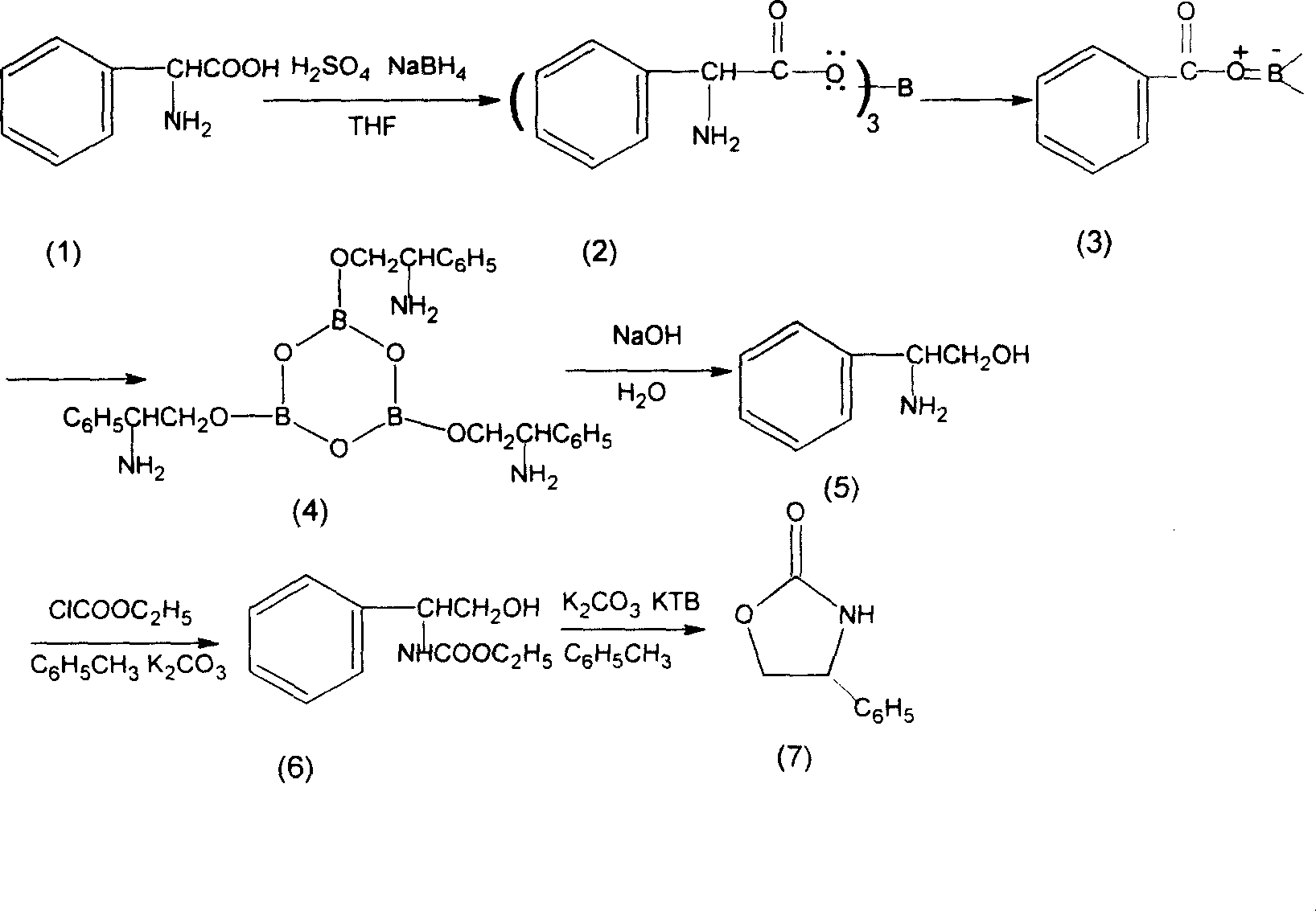
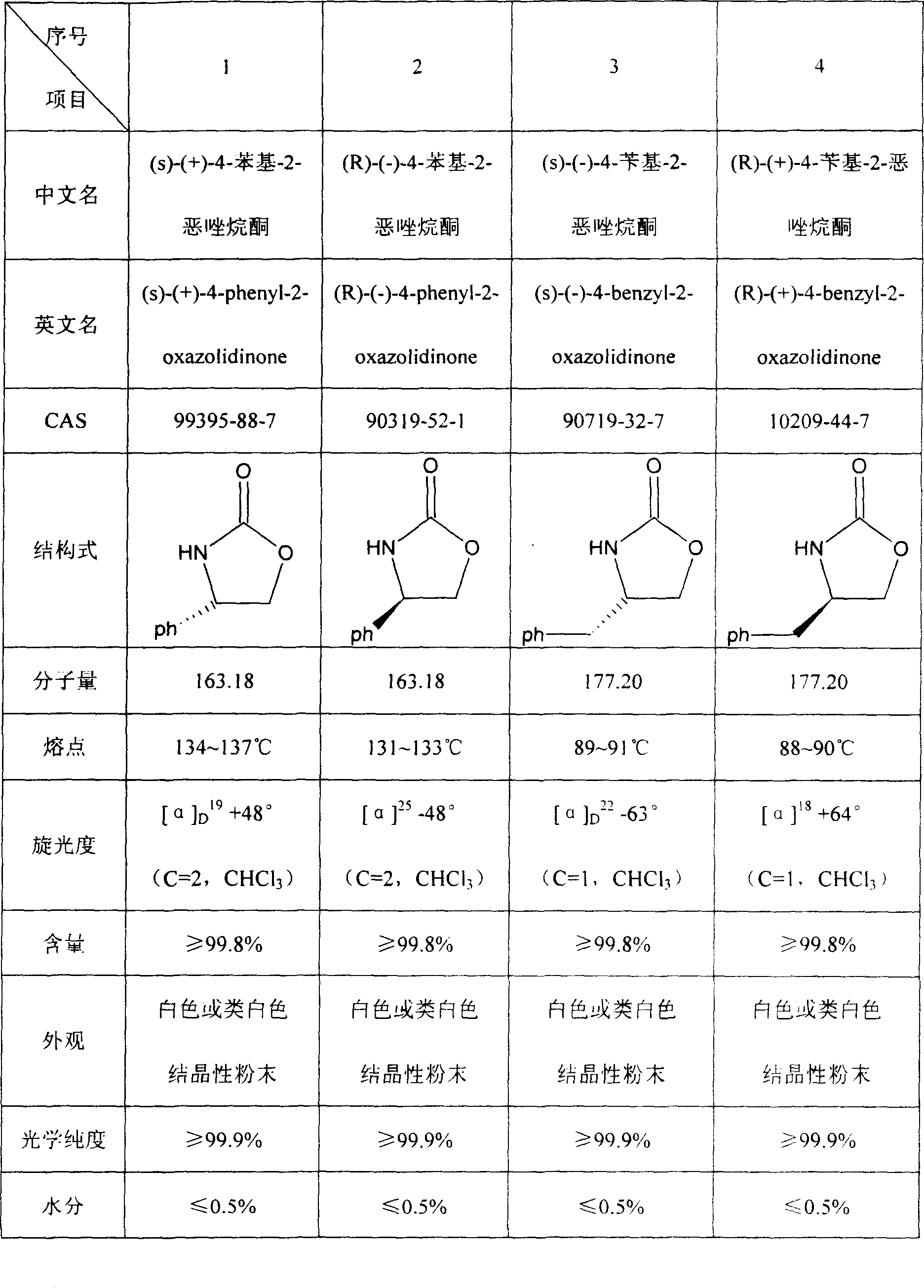
[0021] Example 1 s-(+)-4-phenyl-2-oxazolidinone
[0022] Add 1000l of hydrofuran, 151kg (1kmol) of L-phenylglycine and 95kg of sodium borohydride (2.5kmol) into a 2000l reaction tank, a little heat will be generated, keep at 10-40°C for 4-6 hours after the addition is complete , kept stirring at this temperature for 1 hour, raised the temperature to reflux within 1 hour, refluxed at reflux temperature (about 64-66°C) for 2 hours, evaporated the hydrofuran under normal and reduced pressure, and slowly added 300l water, then add 75kg sodium hydroxide and 300l alkali aqueous solution, heat up to 98-100°C and reflux for 2 hours, after reflux, add 1400l toluene and 200l ethanol for extraction, static layering, separate at 70-80°C Remove the water layer (re-extract the water layer with 300L toluene and combine the organic layers) add 100kg of potassium carbonate to the toluene layer, add 120kg (1.1kmol) of ethyl chloroformate dropwise at 60-80°C, add dropwise for 1 hour, add dropwis...
example 2
[0035] Example 2 (R)-(-)-4-phenyl-2-oxazolidinone
[0036] In the same example 1, the starting material is changed from L-phenylglycine to D-phenylglycine. The yield is 95%, the content is over 99.9%, and the ee value is 99.9%.
example 3
[0037] Example 3 (S)-(-)-4-phenyl-2-oxazolidinone
[0038] In the same example 1, the starting material is L-phenylalanine from L-phenylglycine, the feeding amount is changed from 151kg to 165kg, and the output of the title product is 160kg-168kg. The yield is 90-95%, the content is 99.9%, and the ee value is 99.9%.
[0039] After the reduction reaction of this product, the extraction solvent is a single toluene solvent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com