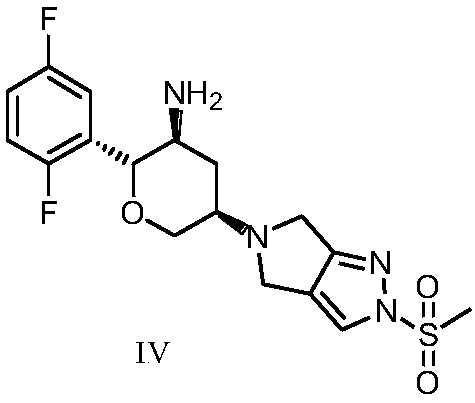
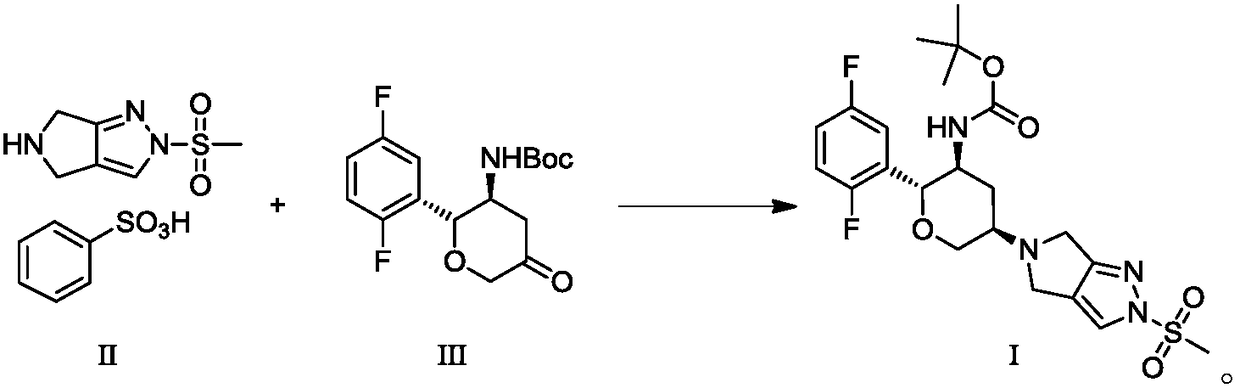
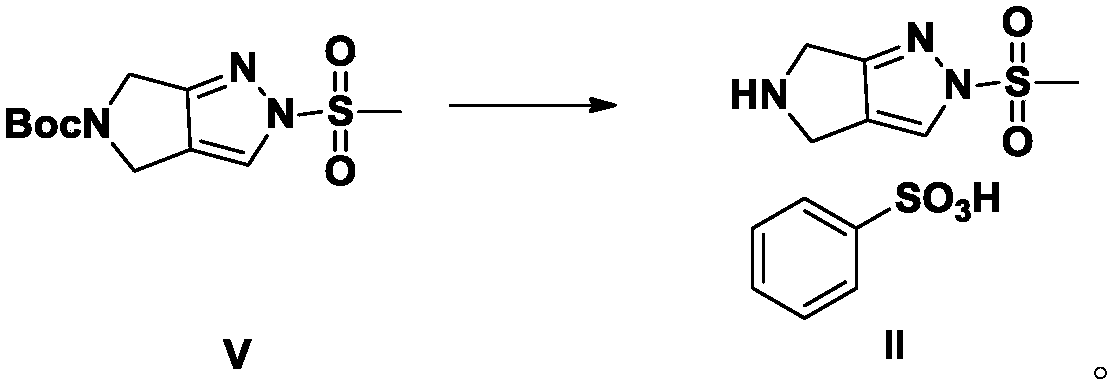
A kind of preparation method of alogliptin and its intermediate
An intermediate and volumetric technology, applied in organic chemistry, bulk chemical production, etc., can solve the problems of unfavorable industrial production, long reaction time, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the preparation method of alogliptin intermediate II
[0049]
[0050] Add 6.65g of benzenesulfonic acid to 30ml of ethyl acetate to dissolve, and cool down to 5-15°C in an ice-water bath; Add 5.75g of pyrrolo[3,4-c]pyrazole to 30ml of ethyl acetate to dissolve it, add it dropwise into the solution of ethyl benzenesulfonate acetate at 5~15℃, stir for 30 minutes, remove from the ice-water bath, and store at 25±5℃ Stir at ℃ for 16 hours, filter with suction, add 35ml ethyl acetate to the filter cake to make slurry, filter with suction, wash the filter cake with 10ml ethyl acetate, dry the filter cake in vacuum at -0.08MPa~-0.1MPa, 40℃ for 10 hours, and get light yellow 6.3 g of off-white solid compound II was obtained, the HPLC purity was 96.52%, and the yield was 91.3%.
Embodiment 2
[0051] Embodiment 2: the preparation method of alogliptin intermediate II
[0052]
[0053] Add 3.3Kg of benzenesulfonic acid to 14L of ethyl acetate to dissolve, and circulate the refrigerated brine bath to drop to 5-15°C; 2.85Kg of )-pyrrolo[3,4-c]pyrazole was dissolved in 14L of ethyl acetate, and added dropwise into ethyl benzenesulfonic acid acetate solution at 5-15°C, stirred for 30 minutes, removed from the ice-water bath, and cooled at 25 Stir at ±5°C for 16 hours, centrifuge, add 17L ethyl acetate to the solid and stir for 1.5 hours; centrifuge, wash the solid with 4L ethyl acetate, dry the wet product at -0.08MPa~-0.1MPa, and vacuum dry at 40°C for 8 hours to obtain a light yellow solid Compound II 3.16Kg, yield 92.3%. HPLC: 96.75%.
Embodiment 3
[0054] Embodiment 3: the preparation method of alogliptin intermediate I
[0055]
[0056] Add 3.27g of compound III and 4.14g of compound II into 20mL N,N-dimethylacetamide, under nitrogen protection, add 2.93g of triethyl orthoformate at 5±5°C, and add 4.23g of sodium acetate borohydride in batches, After the addition, stir in an ice-water bath for 2 hours, heat to 50±5°C and react for about 10 hours; in an ice-water bath, add 60ml of water dropwise below 15°C, stir at this temperature for 1 hour after adding, heat to 50±5°C and stir for 2 hours hour, filter with suction, rinse the filter cake with water three times, add 20ml of water for beating for 1 hour, and filter with suction. Add 16ml of isopropyl ether to the filter cake, heat to 60±5°C, then add 16ml of n-heptane, cool to 15±5°C, keep stirring for 1 hour, filter with suction, -0.08~-0.1MPa, dry in vacuum at 40°C, 3.99 g of a white solid was obtained, with a yield of 80%. LCMS: m / z=499 (M+H) + ; HPLC: 98.62%; C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com