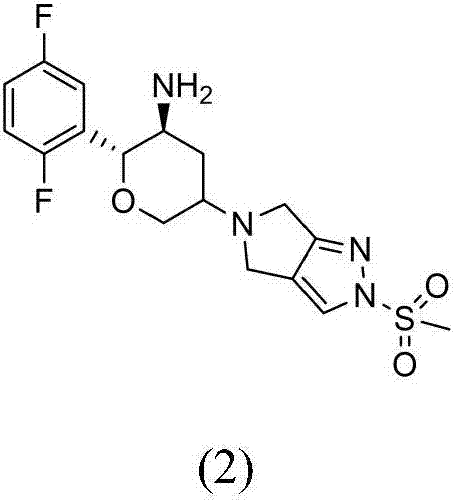
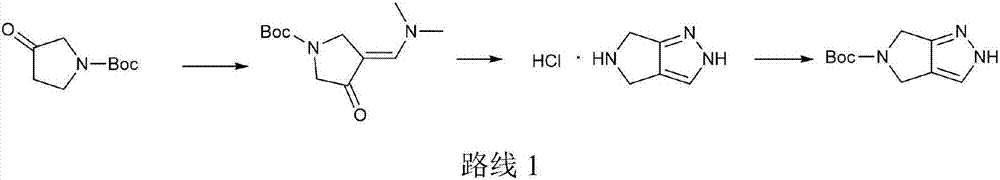
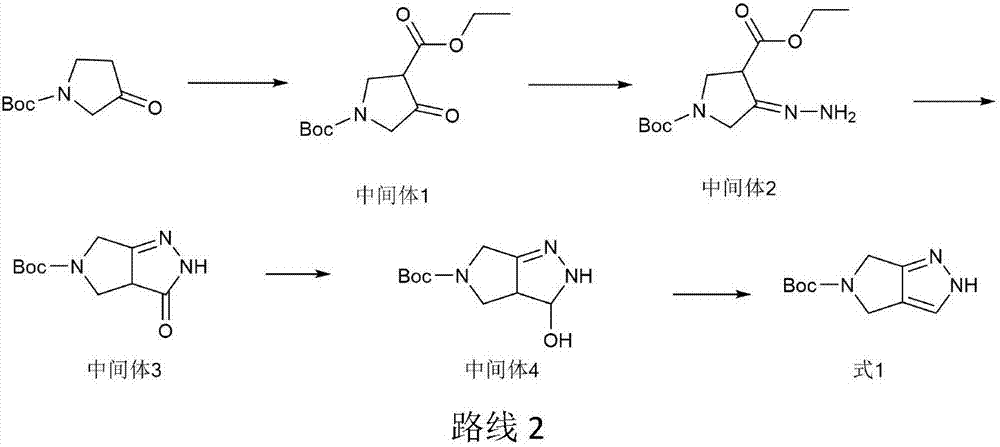
Preparation method for omarigliptin midbody
A technology for intermediates and pyridines, applied in the field of medicine, can solve the problems of potential safety hazards, low yields, easy ignition of low-boiling by-products, etc., and achieves the effects of simple operation, high yields and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of Intermediate 1:
[0032] Add 120g of Boc-pyrrolidone and 600ml of DMF to a 1000ml four-neck flask, stir to cool down to 0-5°C, add 26g of sodium hydride, slowly add 70.5g of ethyl chloroformate dropwise under stirring, and complete the dropwise addition in 40 minutes. After reacting at 0-5°C for 2 hours, after the completion of the reaction detected by TLC, slowly add 3L of ice water, adjust the pH to 7 with dilute hydrochloric acid, extract with ethyl acetate, dry, and concentrate to obtain intermediate 1. Yield 85%.
[0033] Preparation of intermediate 3:
[0034] Add the intermediate 1 obtained above, 500 ml of ethanol to a 1000 ml four-necked flask, and add 45 ml of hydrazine hydrate under stirring at 20-25°C. Stir at 20-25°C for 3 hours. After the reaction is detected by TLC, raise the temperature to reflux temperature of 78°C and continue the reaction for 4 hours. After the reaction is completed, the reaction solution is concentrated, and 500ml of...
Embodiment 2
[0045] Preparation of Intermediate 1:
[0046] Add 120g of Boc-pyrrolidone and 600ml of acetonitrile into a 1000ml four-necked bottle, stir to cool down to 0-5°C, add 30g of sodium hydroxide, slowly add 70.5g of ethyl chloroformate dropwise under stirring, and the dropwise addition is completed in 40 minutes. After reacting at 15°C for 2 hours, TLC detected the completion of the reaction, slowly added 3L of ice water, adjusted the pH to 7.4 with dilute hydrochloric acid, extracted with ethyl acetate, dried, and concentrated to obtain Intermediate 1. Yield 78%.
[0047] Preparation of intermediate 3:
[0048] Add the intermediate 1 obtained above, 500 ml of methanol into a 1000 ml four-necked flask, and add 45 ml of hydrazine hydrate under stirring at 22°C. Stir at 22°C for 3h. After the reaction was detected by TLC, the temperature was raised to 60°C to continue the reaction for 6h. After the reaction was complete, the reaction solution was concentrated, and 500ml of petrole...
Embodiment 3
[0054] Preparation of Intermediate 1:
[0055] Add 120g of Boc-pyrrolidone and 600ml of tetrahydrofuran into a 1000ml four-neck flask, stir to cool down to 20°C, add 52g of potassium carbonate, slowly add 70.5g of ethyl chloroformate dropwise under stirring, and the dropwise addition is completed in 40 minutes. After reacting at ℃ for 2 hours, after the completion of the reaction as detected by TLC, slowly add 3L of ice water, adjust the pH to 6.7 with dilute hydrochloric acid, extract with ethyl acetate, dry, and concentrate to obtain Intermediate 1. Yield 82%.
[0056] Preparation of intermediate 3:
[0057] Add the intermediate 1 obtained above, 500 ml of acetonitrile into a 1000 ml four-necked flask, and add 45 ml of hydrazine hydrate under stirring at 24°C. Stir at 24°C for 3h. After the reaction was detected by TLC, the temperature was raised to reflux temperature of 78°C to continue the reaction for 4h. After the reaction was completed, the reaction solution was conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com