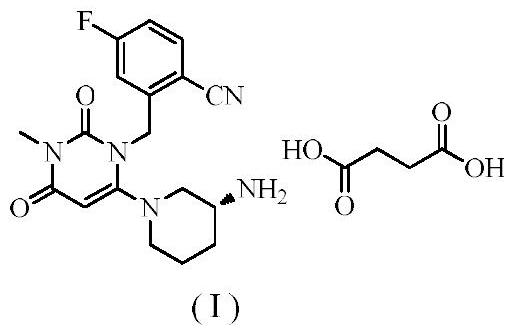
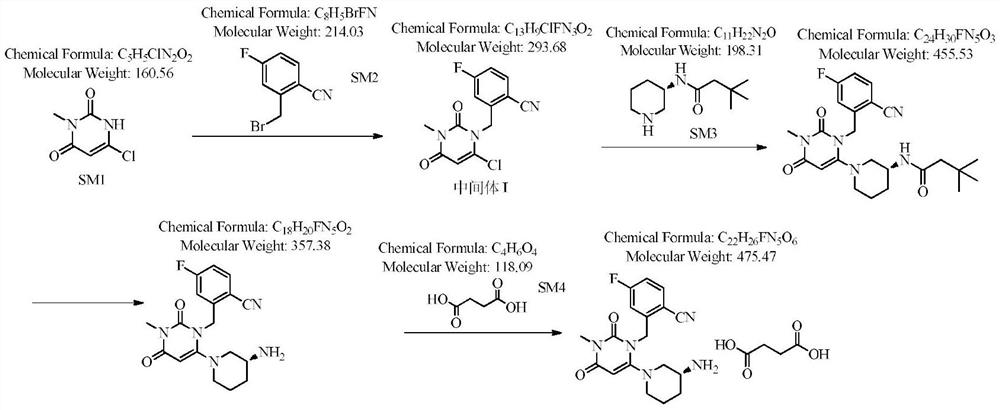
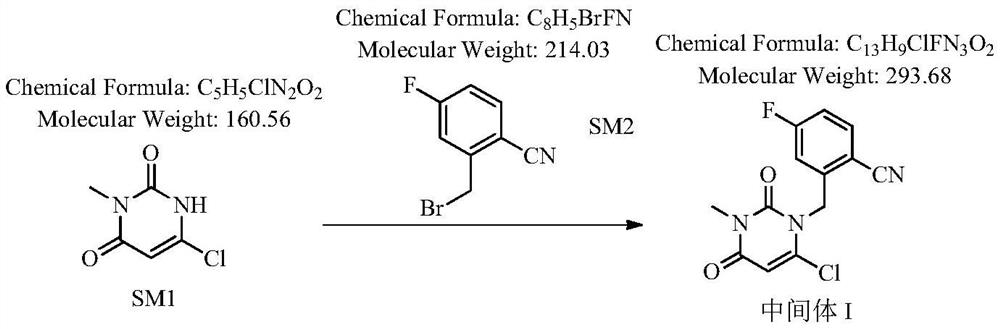
Novel preparation process of trelagliptin succinate
A one-pot technology of troxagliptin succinate, applied in the field of drug synthesis, can solve problems such as labor costs, waste water defects, and three waste treatment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Step 1: Preparation of 2-[(6-chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]-4-fluorobenzonitrile
[0041] Add 100 g of 3-methyl-6-chlorouracil, 140 g of 2-cyano-5-fluorobenzyl bromide (1.05 equivalents), 800 ml of toluene, and 38.4 g of potassium hydroxide (1.1 equivalents) into the reaction flask. The reaction was stirred at 40-50° C., detected by TLC, and the reaction was complete in 3 hours, which was directly used for the next reaction.
[0042] Step 2: Preparation of Boc-Trexagliptin
[0043] Add 113.2 g of (R)-3-Boc-aminopiperidine dihydrochloride (1.05 equivalents) to the reaction flask of the first step above, stir and react at 40-50° C., detect by TLC, and complete the reaction in 2 hours. After filtering, the solvent of the filtrate was evaporated to dryness to obtain Boc-trexagliptin.
[0044] Step 3: Preparation of Trexagliptin
[0045] Add the above-mentioned Boc-trexagliptin to 400 ml of trifluoroacetic acid, stir and react at 30-40° C....
Embodiment 2
[0049] Different from Example 1: the first and second reaction solvents are N-dimethylformamide (DMF), the total yield of the four-step reaction is 75.2%, and the HPLC purity is 99.73%.
Embodiment 3
[0051]Different from Example 1: the first and second reaction solvents are N-methylpyrrolidone (NMP), the total yield of the four-step reaction is 75.5%, and the HPLC purity is 99.76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com