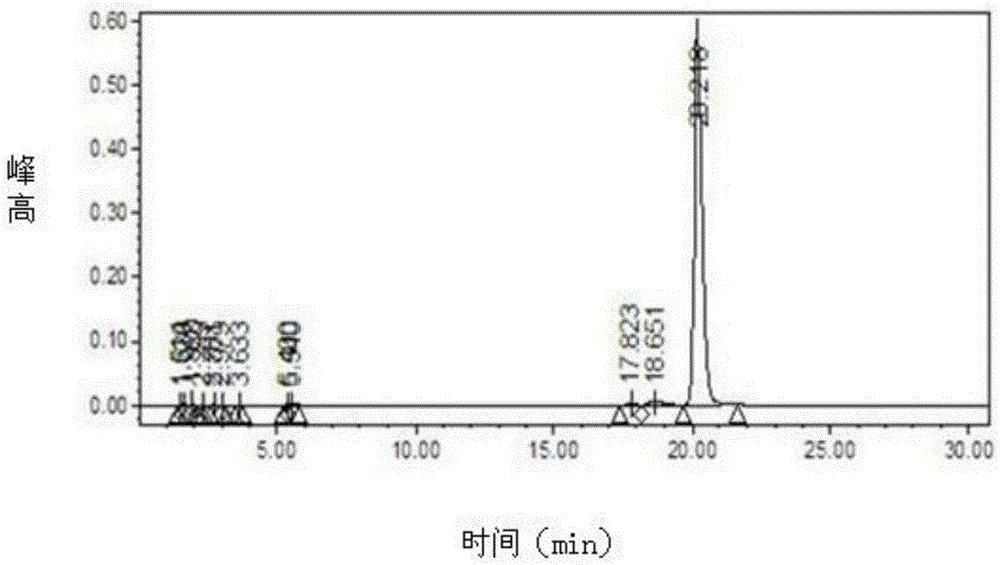
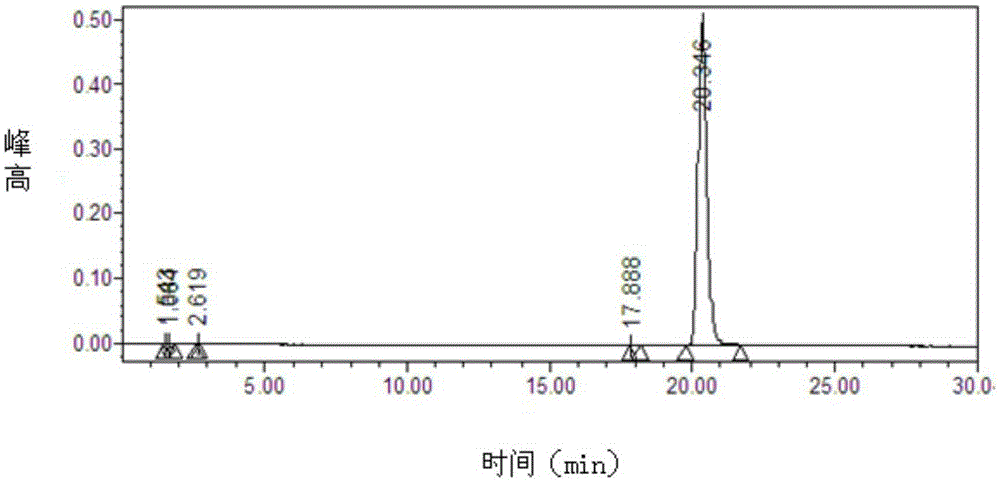
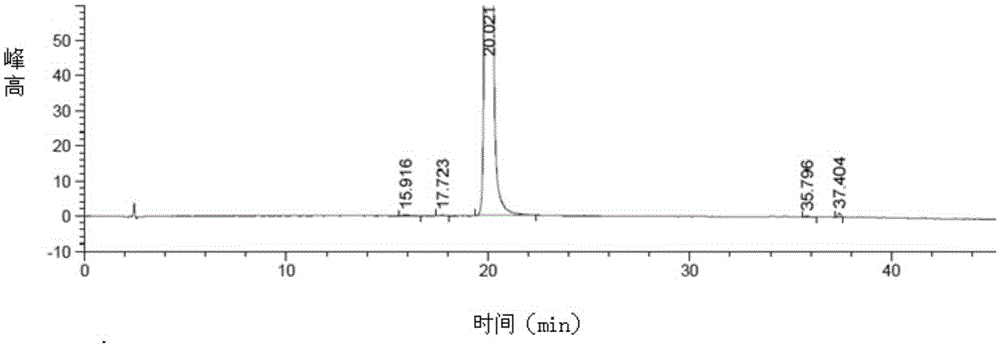
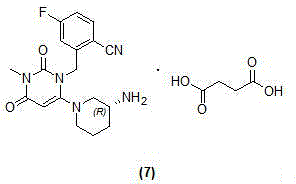
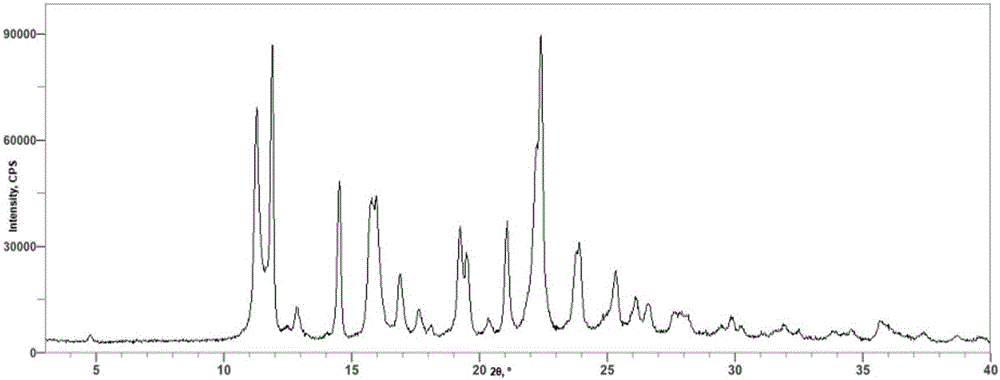
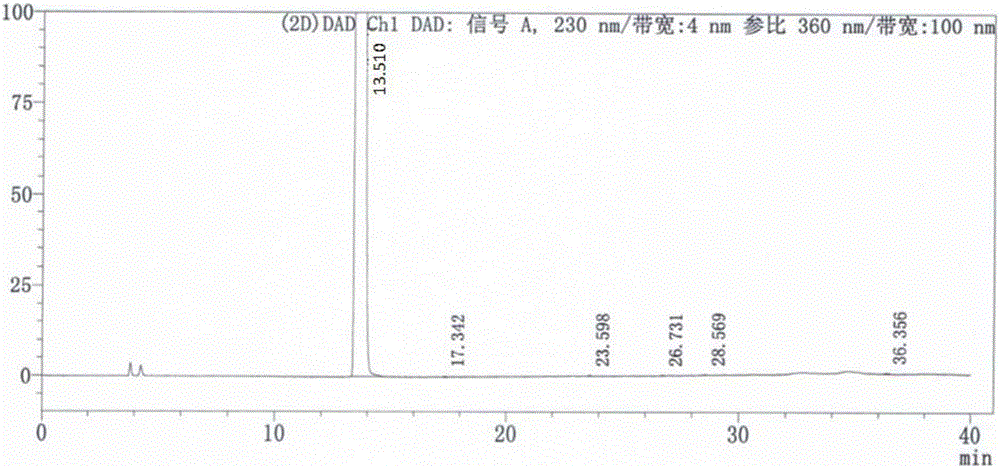
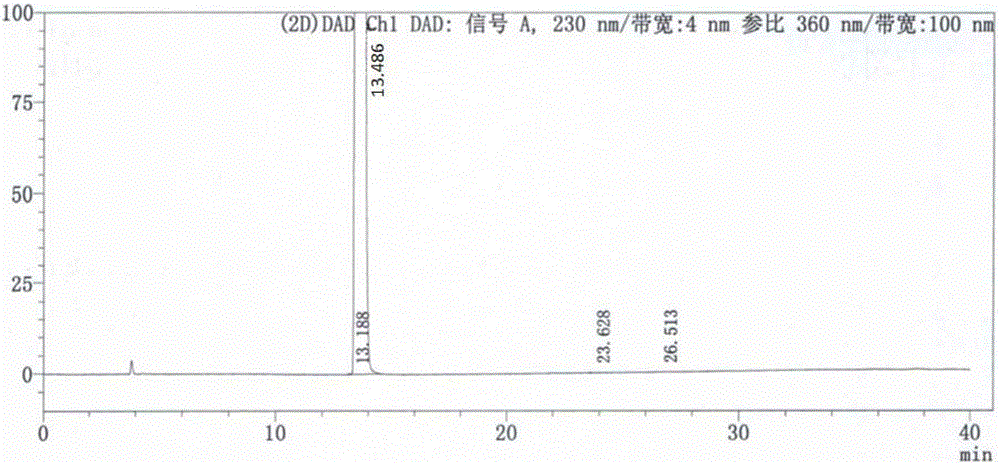
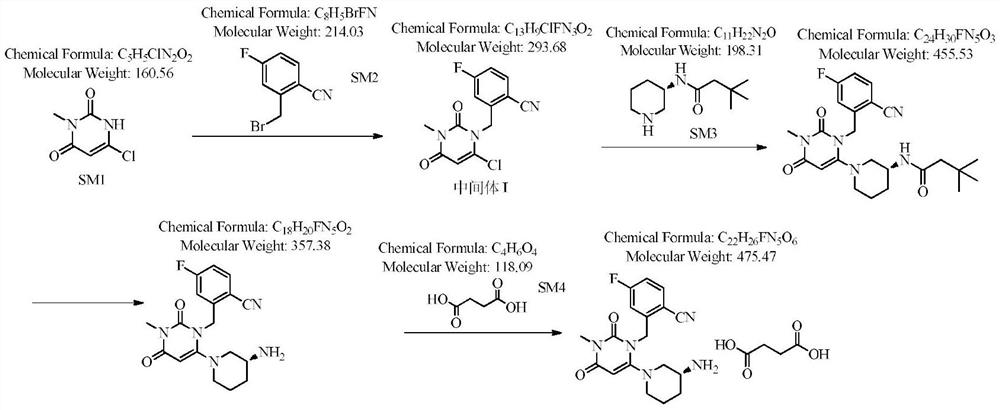
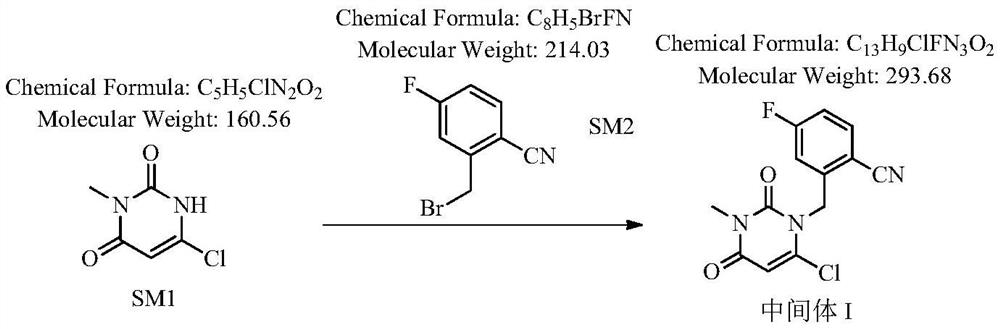
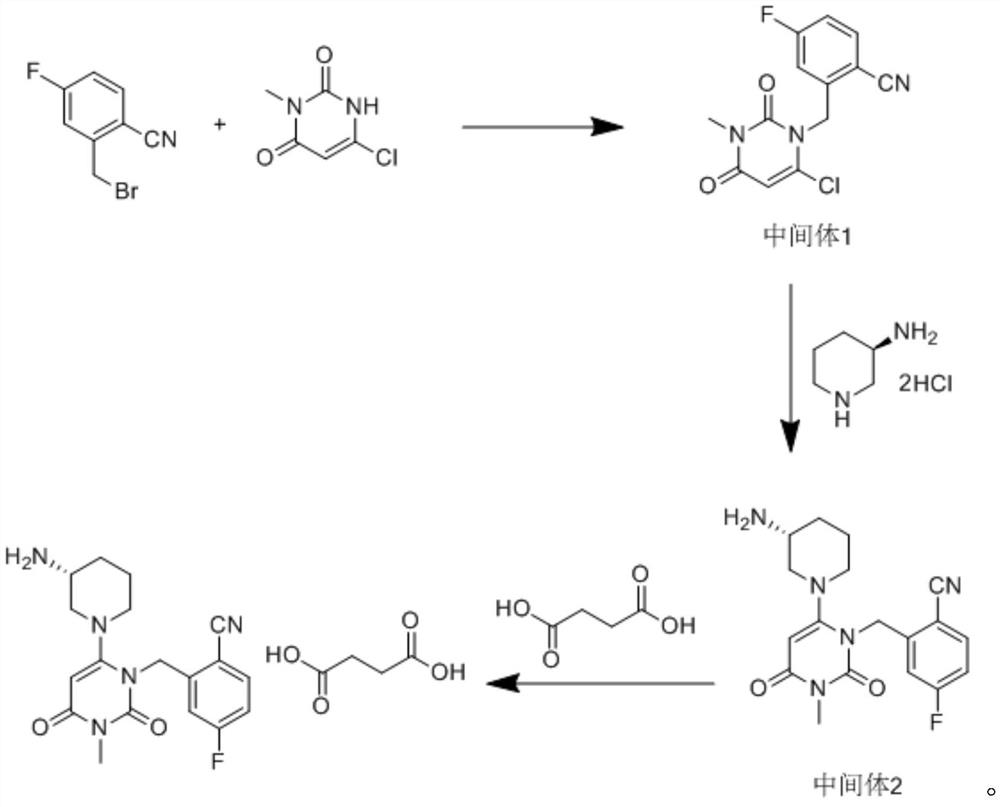
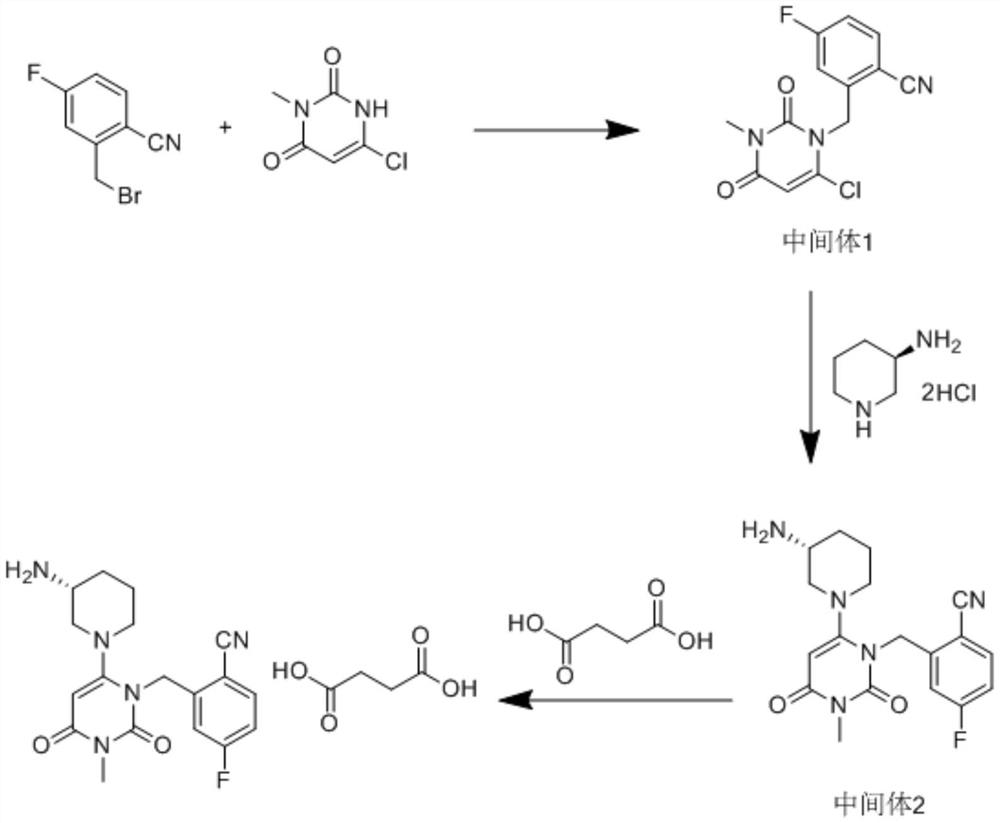
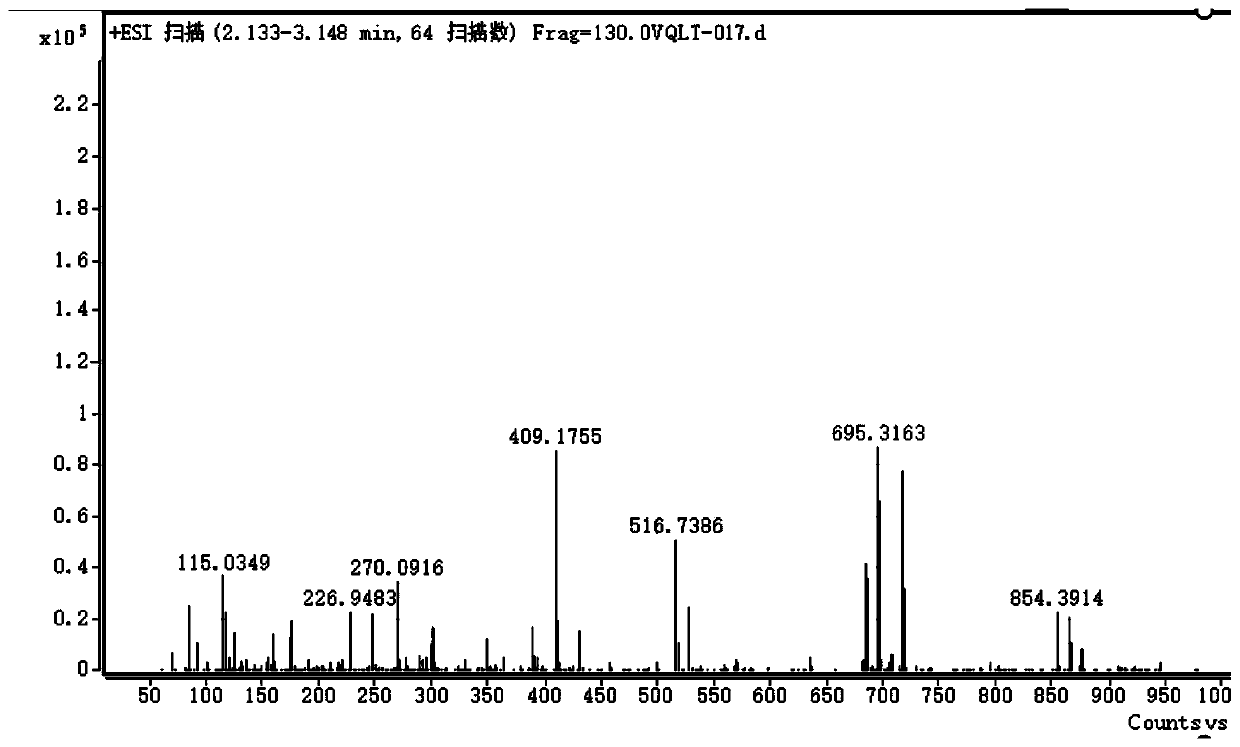
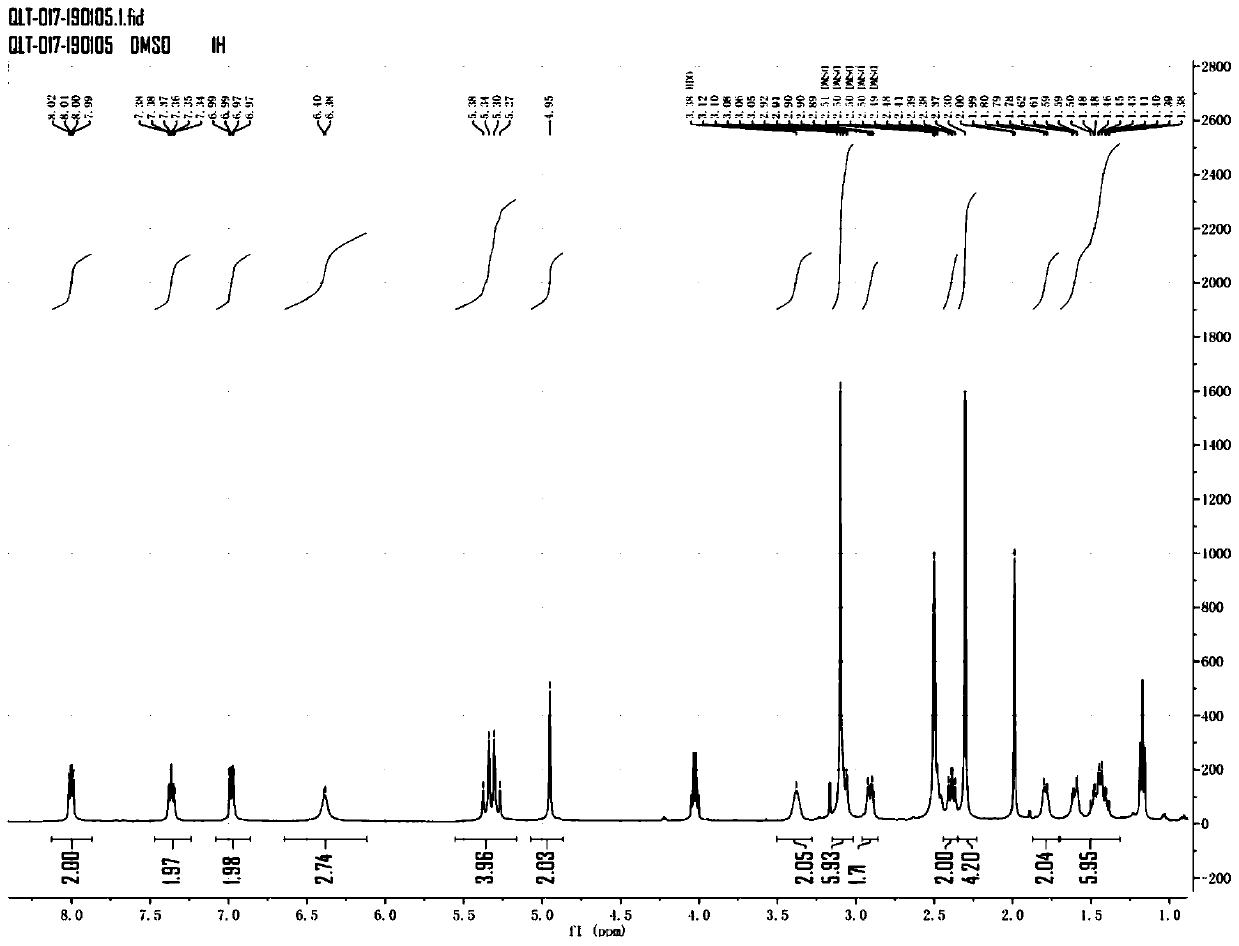
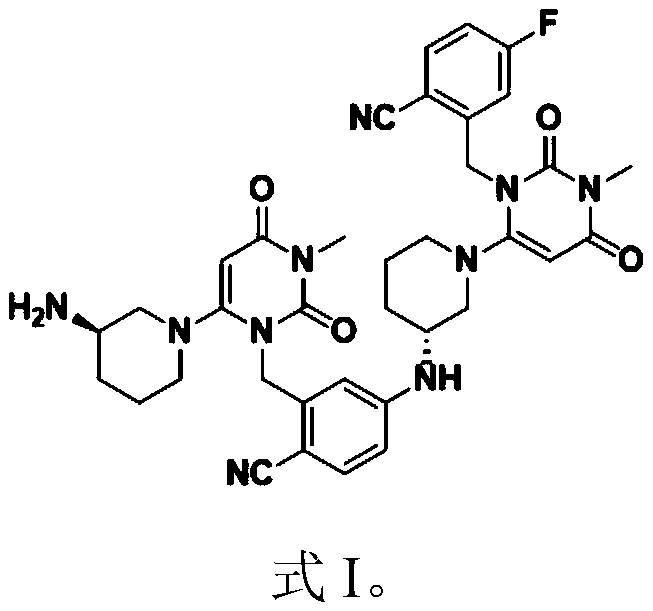
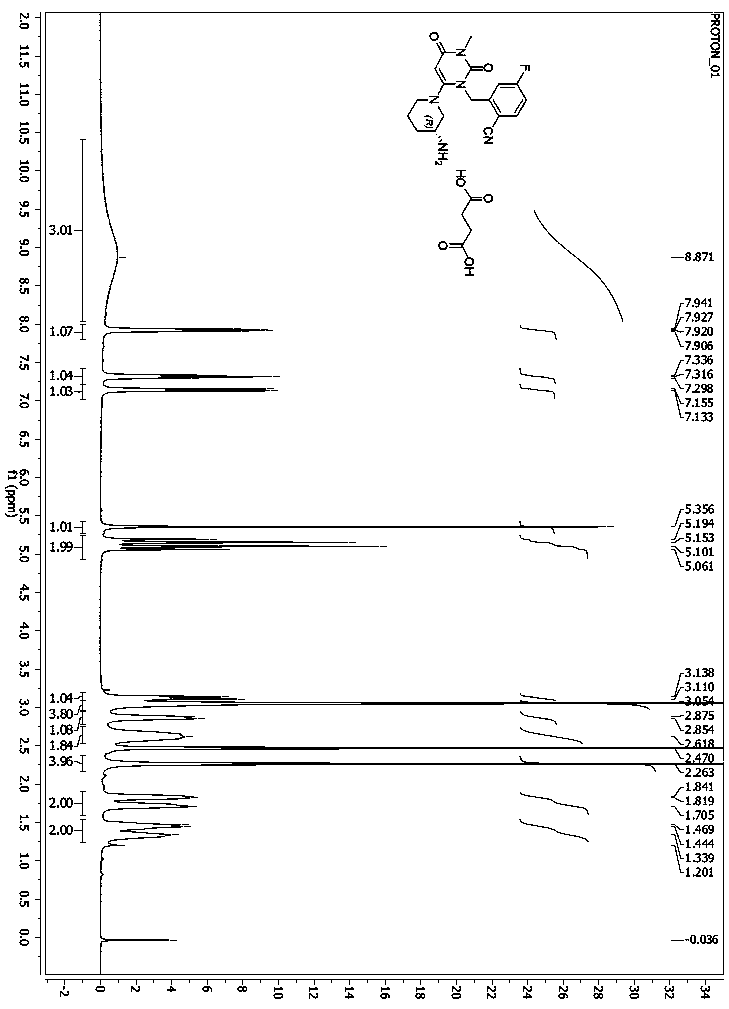
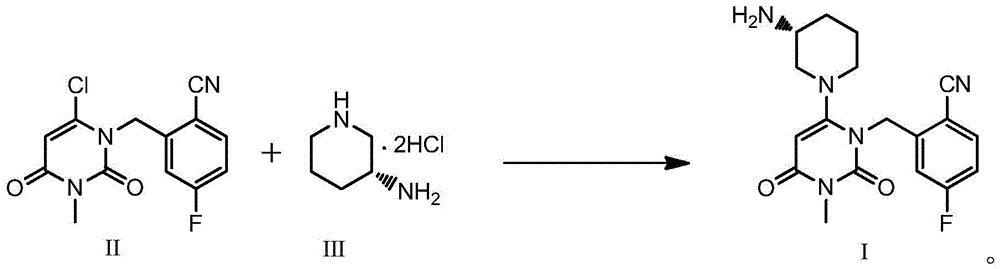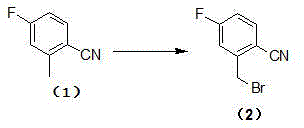Patents
Literature
43 results about "Trelagliptin succinate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
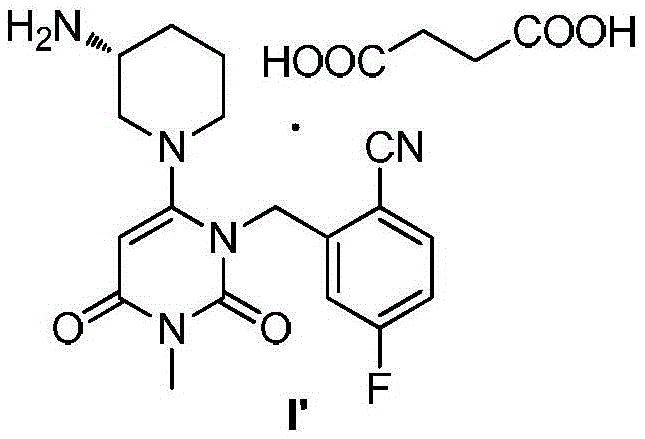
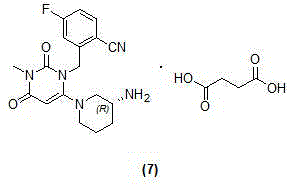
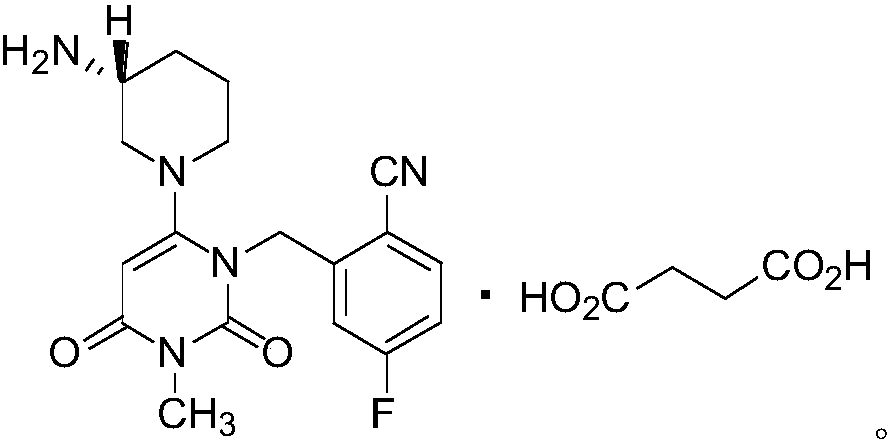
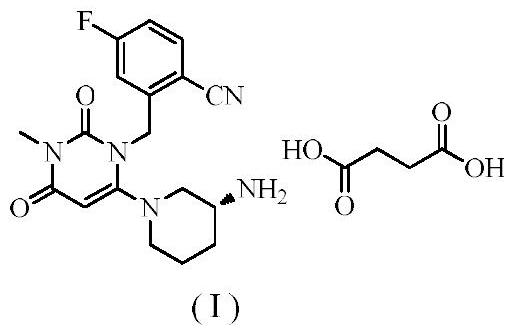
Trelagliptin (Zafatek) is a pharmaceutical drug used for the treatment of type 2 diabetes (diabetes mellitus). Indications. It is a highly selective dipeptidyl peptidase-4 inhibitor that ... Formulated as the salt trelagliptin succinate, it was approved for use in Japan in March 2015.
Related substance detection method for trelagliptin succinate and preparation thereof
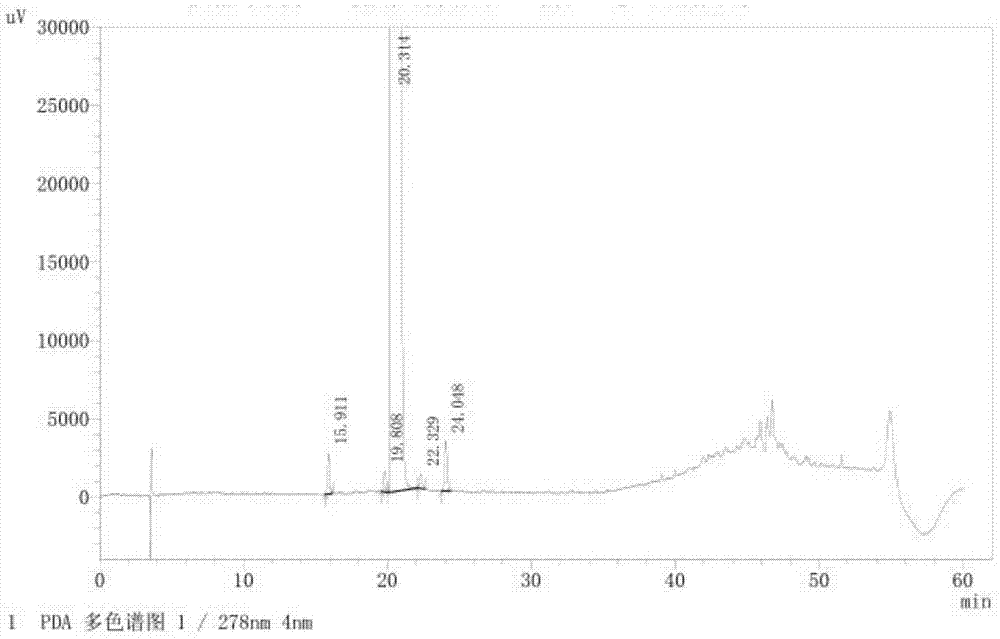
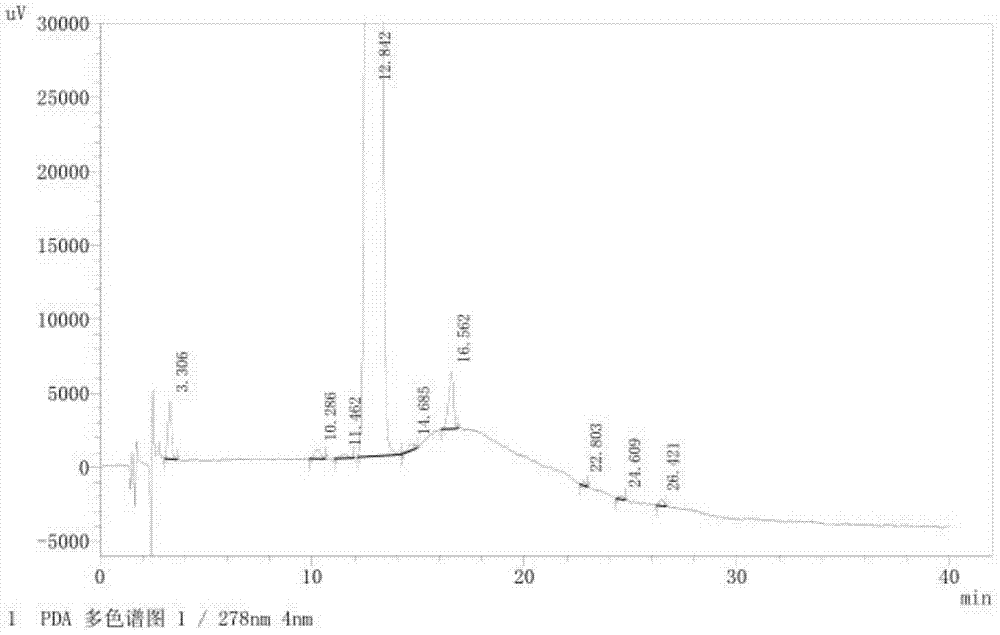
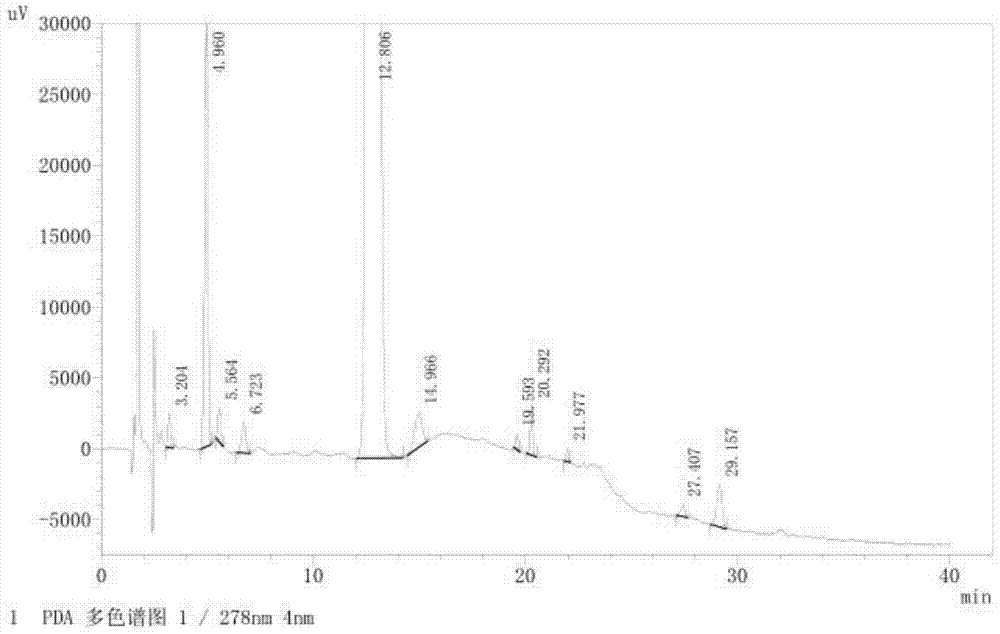
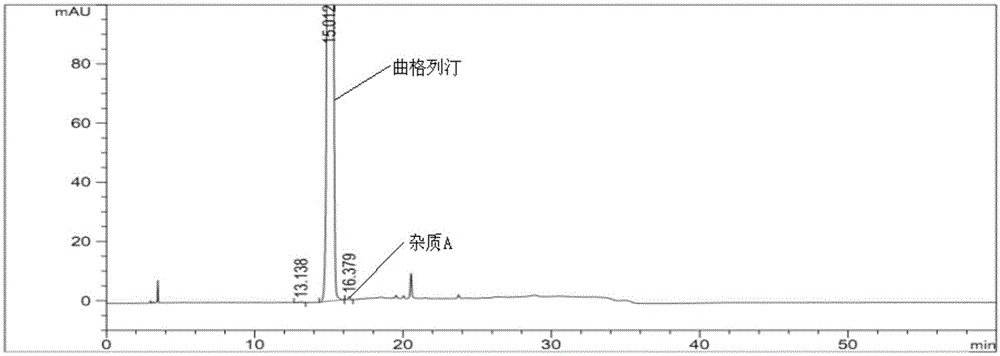
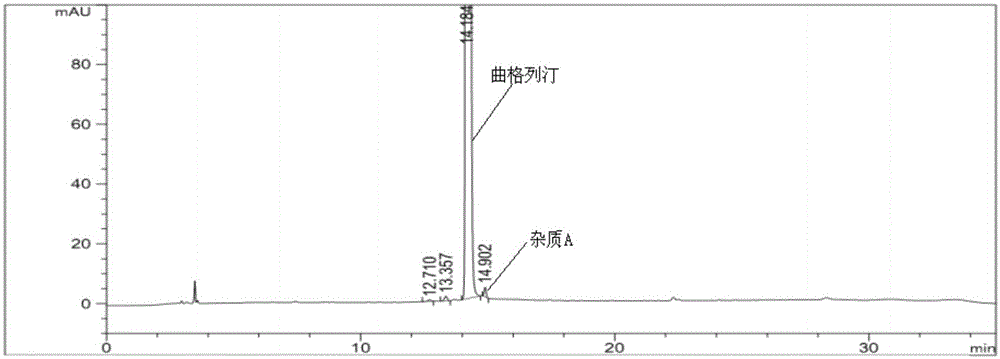
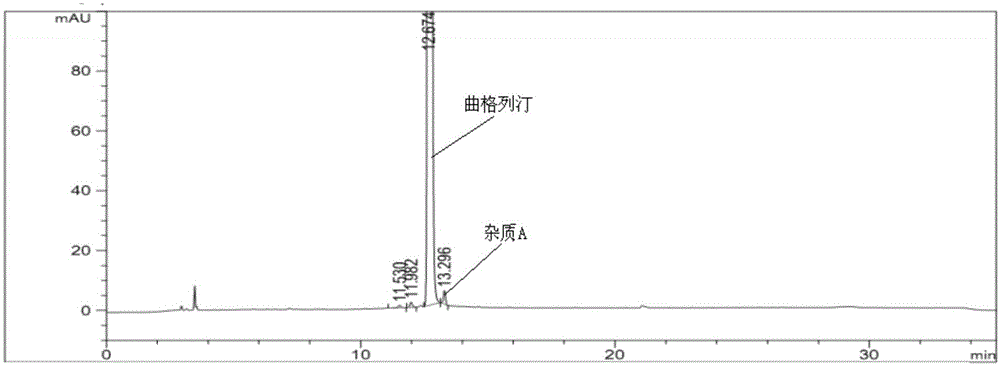
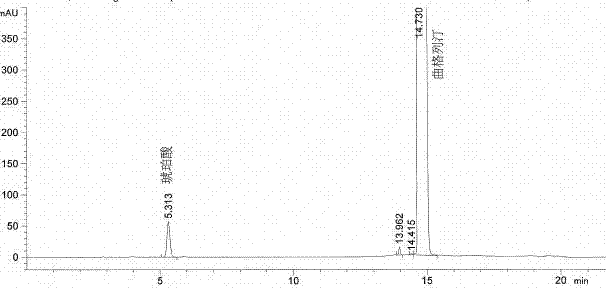
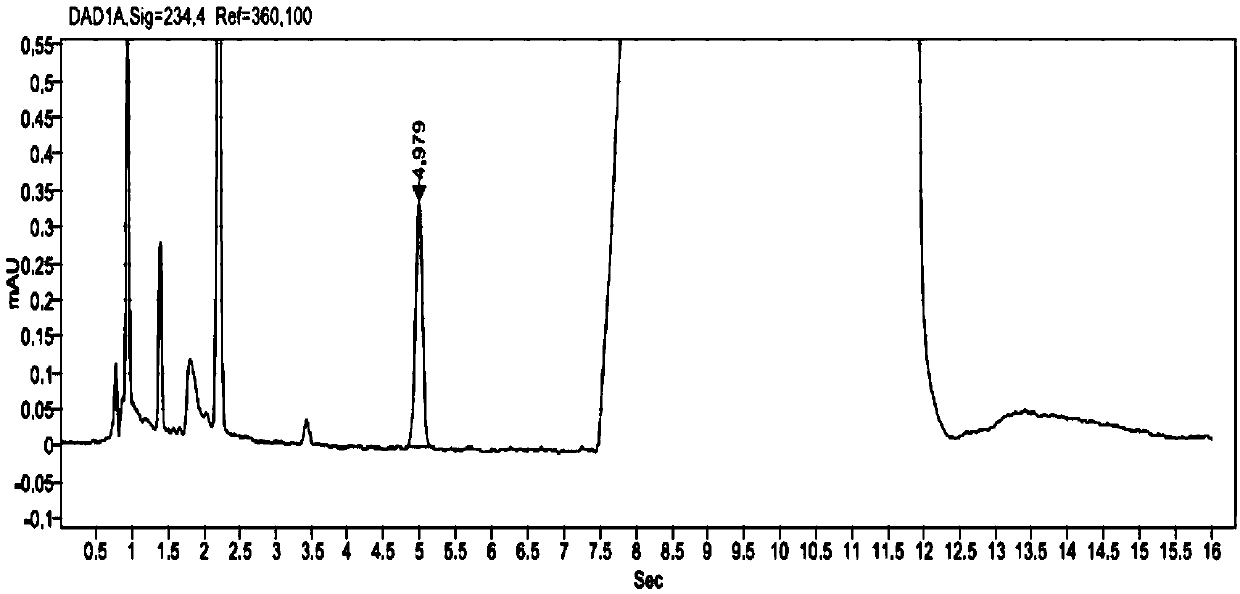
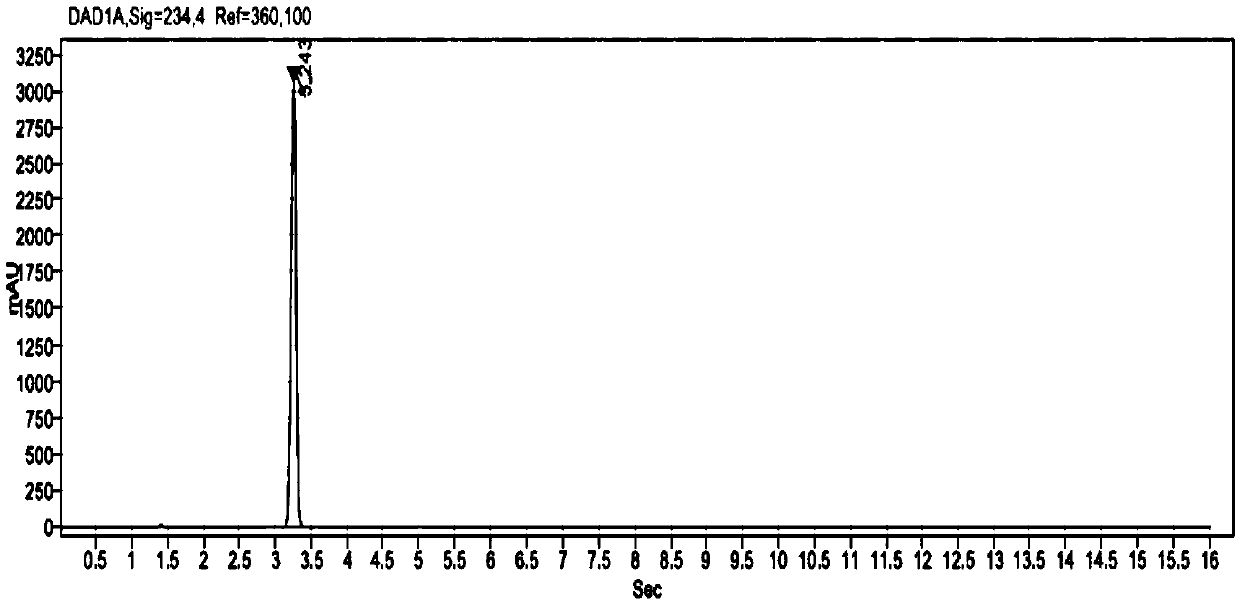
The invention provides a related substance detection method for trelagliptin succinate and preparation thereof. According to the related substance detection method for trelagliptin succinate and preparation thereof, a diode array detector is adopted, a chromatographic column adopted in the method is an octadecyl silane bonded silica gel chromatographic column, a mobile phase A is phosphate buffer solution, a mobile phase B is acetonitrile, the volume ratio of the phosphate buffer solution to acetonitrile is 60: 40 to 85: 15, the pH value of the phosphate buffer solution is 5.0 to 5.5, the concentration of the phosphate buffer solution is 0.05 to 0.1mol / L, and a detection wavelength is 278nm. The various ingredients in the analysed substances are thorough in separation and high in operability; the analysis method is high in specificity, high in accuracy and sensitivity, high in adaptability, and capable of providing a basis for the safety, effectiveness and controllability of medicines.
Owner:JINAN ORIENT KAIYUAN PHARMA TECH
Oral tablet containing trelagliptin succinate and preparation method thereof
ActiveCN104825413AGood effectSuitable for commercial productionOrganic active ingredientsMetabolism disorderFiller ExcipientSilica gel
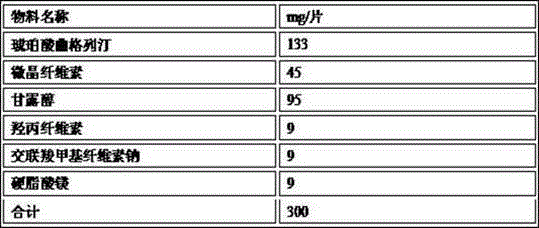
The invention provides an oral tablet containing trelagliptin succinate and a preparation method thereof. The tablet do not contain flow aids such as micro-powder silica gel and comprises 35-50 percent by weight of trelagliptin succinate, the trelagliptin succinate tablet cores are prepared by tabletting trelagliptin succinate granules and lubricating agents, and filling agents only exist in the tabletting trelagliptin succinate granules. The trelagliptin succinate tablet prepared by the method disclosed by the invention is stable and controllable in quality, the poor phenomena such as low liquidity in the granulation process and phenomena such as sticking, adhesion, tablet capping and layering during tabletting are avoided, and the oral tablet is suitable for commercialized production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Trelagliptin and preparation method of succinate thereof
ActiveCN105669645AReduce contentSimple and safe operationOrganic chemistry methodsCarboxylic acid salt preparationOrganic solventPhosphate
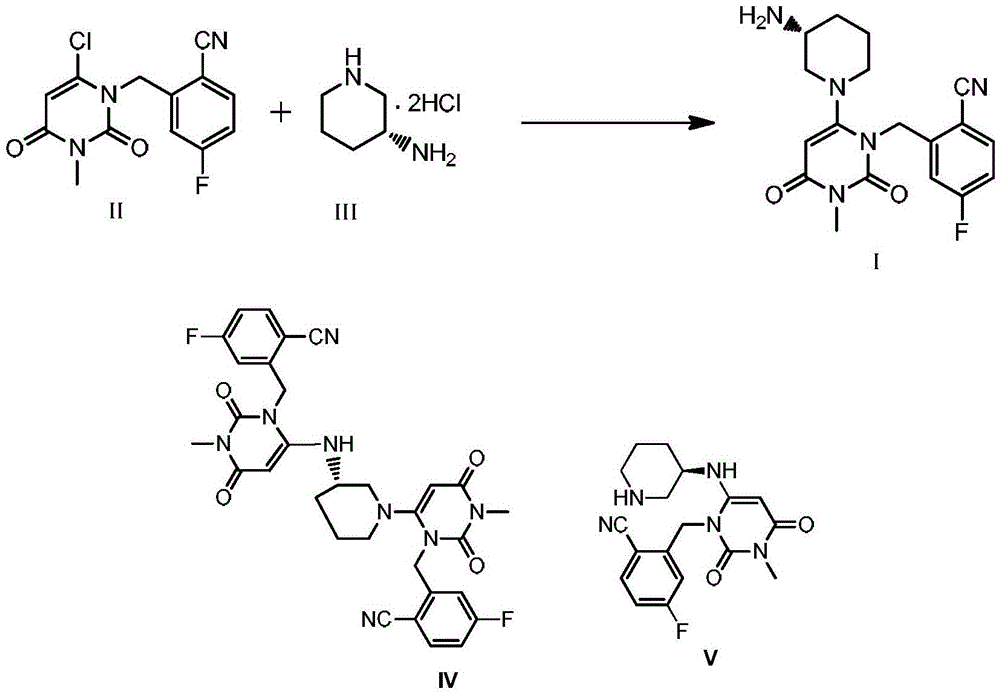
The invention discloses a trelagliptin and a preparation method of succinate thereof. The preparation method includes the following steps that a trelagliptin intermediate II and a trelagliptin intermediate III perform nucleophilic substitution reaction in an organic solvent in the presence of phosphate and a phase transfer catalyst to obtain trelagliptin I, and then trelagliptin I reacts with the succinate to produce salt. The operation process of the preparation method is simple and safe, special devices are not needed, the impurity contents of bis-substituted and primary amine substituted isomers can significantly reduced, only one-time recrystallization is needed, the purity of the obtained trelagliptin succinate I' can be above 99.5%, the impurity contents of the bis-substituted and primary amine substituted isomers are both below 0.05%, the contents of all the other impurities are all below 0.05%, the prepared trelagliptin succinate I' is high in purity, the production cost is low, and the trelagliptin and the preparation method are suitable for industrialized production.
Owner:上海新礼泰药业有限公司
Preparation method for trelagliptin succinate
The invention discloses a preparation method for trelagliptin succinate. The method uses raw materials which are low in toxicity and are small in pollution. The preparation method adopts the following synthesis route as shown in the description. The preparation method is used for preparing the trelagliptin succinate which is relatively high in yield and is relatively high in purity. The preparation method for the trelagliptin succinate is high in safety, is gentle in reaction condition, is high in yield and is high in purity, obviously reduces impurities in a target product, and reduces production cost, and is beneficial for industrial production.
Owner:ZHENGZHOU MINGZE MEDICAL TECH
Analytic method for related substances in raw materials and preparation of trelagliptin succinate
ActiveCN106896166AQuick monitoringEffective monitoringComponent separationColor/spectral properties measurementsIsocratic elutionPhosphate
The invention discloses an analytic method for related substances in raw material and a preparation of trelagliptin succinate. A high performance liquid chromatography is adopted, and chromatographic conditions include that a chromatographic column is octadecyl silane bonded silica gel chromatographic column, the mixed solution with the pH of 3.3-3.7 and the volume ratio of phosphate buffer aqueous solution to acetonitrile of (88-92):(8-12) is taken as a mobile phase A, the mixed solution with the pH of 3.3-3.7 and the volume ratio of phosphate buffer aqueous solution to acetonitrile of (18-22):(78-82) is taken as a mobile phase B, detection wavelength is 218-222nm and isocratic elution is carried out. The analytic method disclosed by the invention can detect many impurities and can rapidly, effectively and accurately monitor the related substances in trelagliptin succinate.
Owner:合肥拓锐生物科技有限公司
Preparation method of trelagliptin succinate
InactiveCN106632241AExtended reaction timeSave materialOrganic chemistryChemistryTrelagliptin succinate
The invention discloses a preparation method of trelagliptin succinate. The preparation method comprises four steps. The method for preparing the trelagliptin succinate is rapid and efficient. By adopting the method disclosed by the invention, reaction time is shortened and materials are saved.
Owner:安徽省金楠医疗科技有限公司
Preparation method of trelagliptin succinate
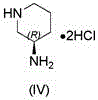
The invention relates to the technical field of drug preparation, and discloses a preparation method of trelagliptin succinate. The preparation method comprises the following steps: performing a reaction on a compound represented by a formula (2), (R)-3-amino-piperidine dihydrochloride and a weak base in a molar ratio of 1: (1-2): (1.5-5) to obtain a coarse product represented by a formula (3); and then recrystallizing the coarse product represented by the formula (3) by using an organic solvent with medium polarity to remove main impurities close to the coarse product represented by the formula (3) in polarity. The preparation method solves the problems in an existing preparation method that the difficulty for separating and purifying a final product is great, the yield and purity are low, the amount of the organic solvent during separation and purification is large and green industrial scaled production is not facilitated. The preparation method disclosed by the invention is high in total yield, and the purity of trelagliptin succinate is 99.83%, so that safe medication of trelagliptin succinate is guaranteed, and the quality and safe medication of trelagliptin succinate are improved.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
Industrialization-suitable preparation method of high-purity trelagliptin succinate
ActiveCN105315256AMild reaction conditionsLess impuritiesCarboxylic acid salt preparationBulk chemical productionAfter treatmentEngineering
The invention relates to a novel industrialization-suitable preparation method of high-purity trelagliptin succinate and aims to solve the problem of generation of much intermediate impurity, complex after treatment and high cost in the prior art. The invention discloses the preparation method of the trelagliptin succinate, which is different from the reported preparation methods. The preparation method of the trelagliptin succinate is mild in conditions, is environment-friendly, is high in conversion rate and is high in finish product quality.
Owner:REGENEX PHARMA LTD
Technological improvement method for preparing trelagliptin succinate
ActiveCN106279104AReduce pollutionOvercome the shortcomings of cumbersome industrial operationsOrganic chemistrySolventReaction step
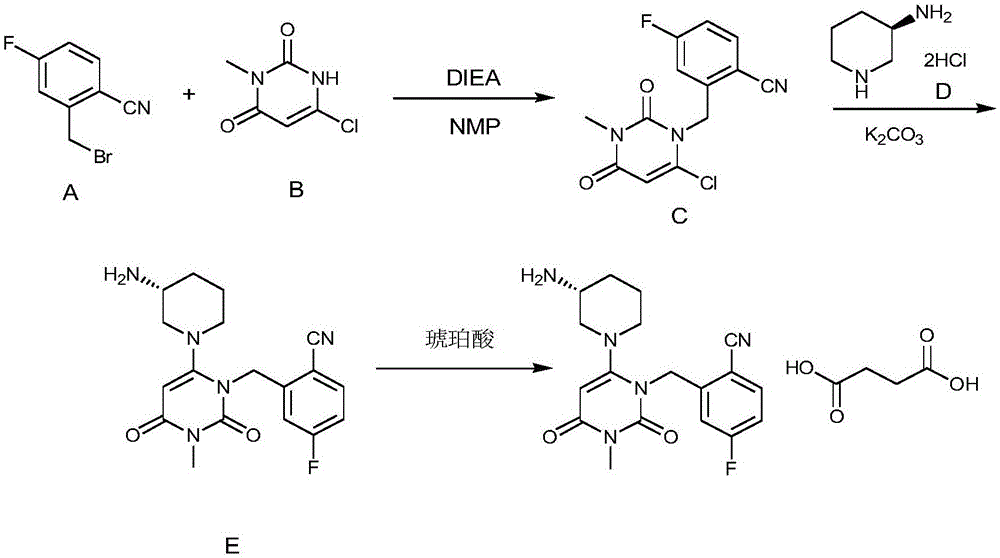
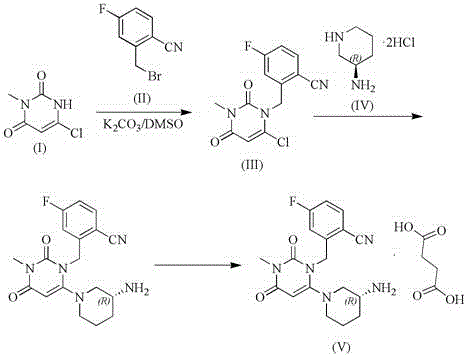
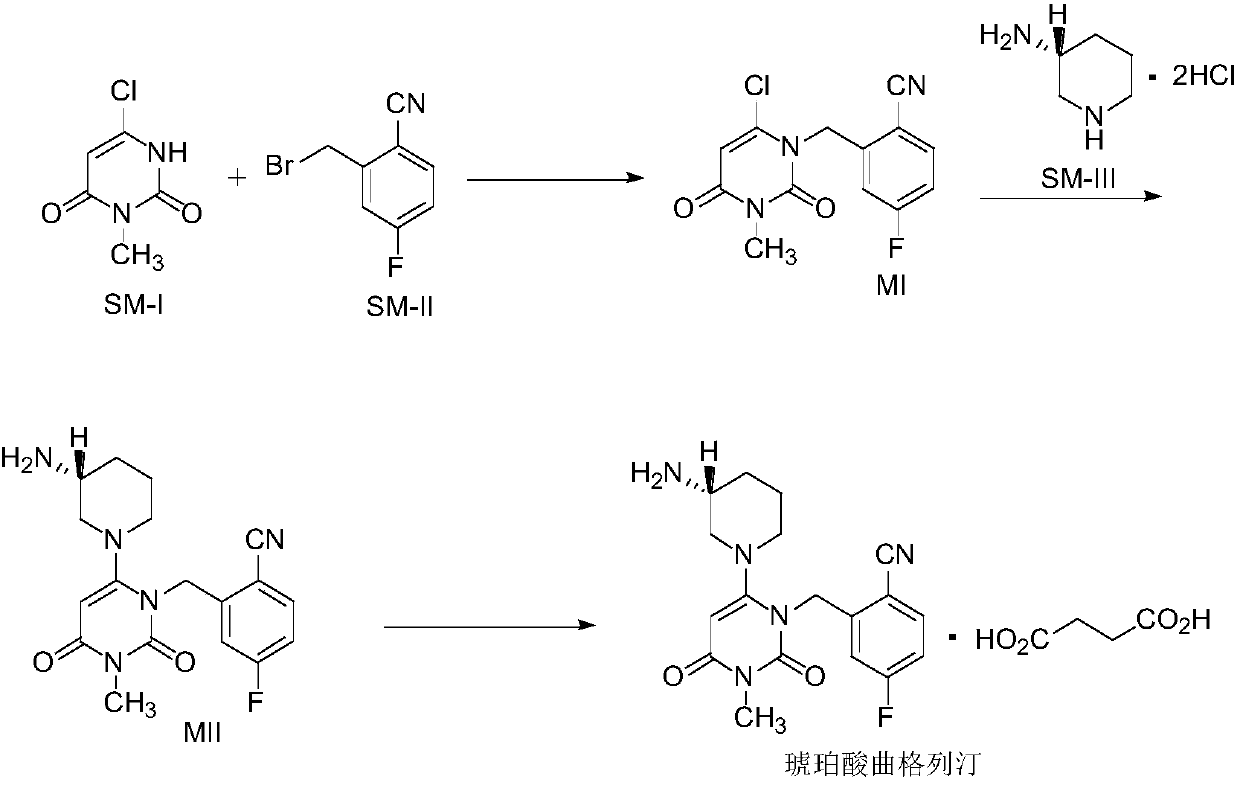
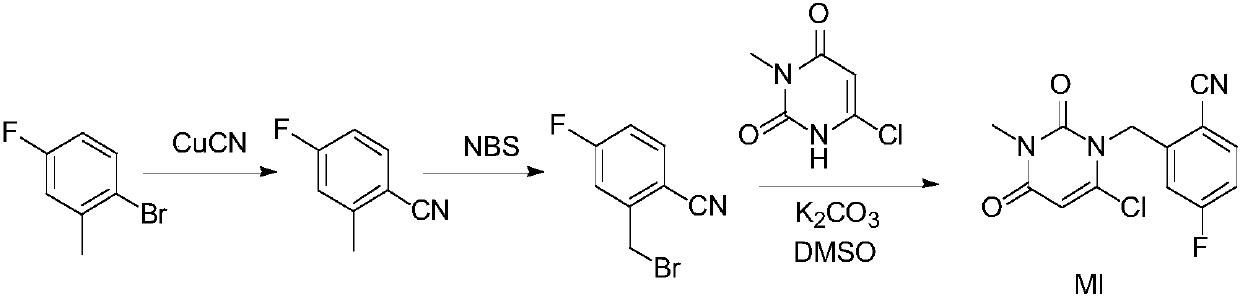
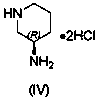
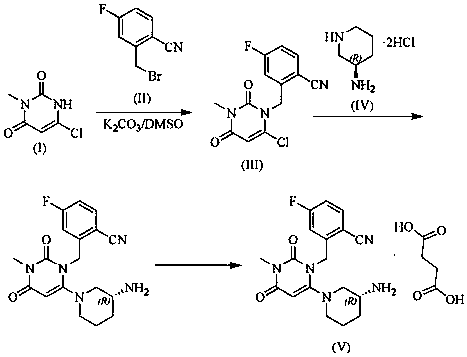
The invention relates to a technological improvement method for preparing trelagliptin succinate (compound V). The method comprises the following steps: by taking 6-chloro-3-methyluracil in formula (I) shown in the description and 2-cyano-5-fluorobenzyl in formula (II) shown in the description as raw materials, carrying out bromo benzyl condensation reaction to obtain a compound III, selecting an appropriate solvent and control reaction condition, enabling a midboy III to be directly subjected to piperidine condensation reaction with (R)-3-aminopiperidine dihydrochloride shown in formula (IV) without separation and purification, and finally carrying out a salifying one-pot method to prepare trelagliptin succinate (compound V). Trelagliptin succinate is prepared by using the one-pot method, so that the reaction steps are reduced, the operation process is simplified, the production efficiency is improved, and the method is safe, environmentally friendly and suitable for the industrialized production.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
Method for separating and analyzing trelagliptin succinate and preparation related substances thereof
InactiveCN106290596AEfficient separationGood elution separation effectComponent separationStationary phaseOrganic solvent
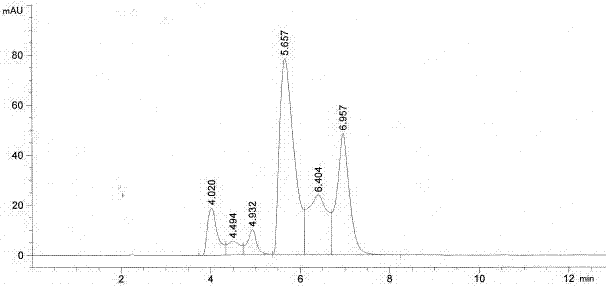
The invention discloses a method for separating and analyzing trelagliptin succinate and preparation related substances thereof. According to the method, the chromatographic conditions comprise that an octadecyl bonded silica gel is adopted as a stationary phase, a buffer liquid is adopted as a mobile phase A and an organic solvent is adopted as a mobile phase B to carry out gradient elution, and the gradient elution conditions comprise that the mobile phase A is 85-50 (V%) and the mobile phase B is 15-50 (V%) at the time of 0-10 min, the mobile phase A is 50 (V%) and the mobile phase B is 50 (V%) at the time of 25-30 min, and the mobile phase A is 85-50 (V%) and the mobile phase B is 15-50 (V%) at the time of 31-35 min; the sample solution preparation comprises that the sample to be detected is prepared into the sample solution by using the mobile phase A; and separating and analyzing are performed, wherein the sample solution is injected into a high performance liquid chromatography so as to complete the detection of the trelagliptin succinate and the preparation related substances thereof. With the method of the present invention, the trelagliptin and the related substances can be rapidly and effectively separated under the same chromatographic conditions. The detection method of the present invention has advantages of high specificity, high precision, strong accuracy, convenient operation, and effective control of drug quality.
Owner:WATERSTONE PHARMA WUHAN
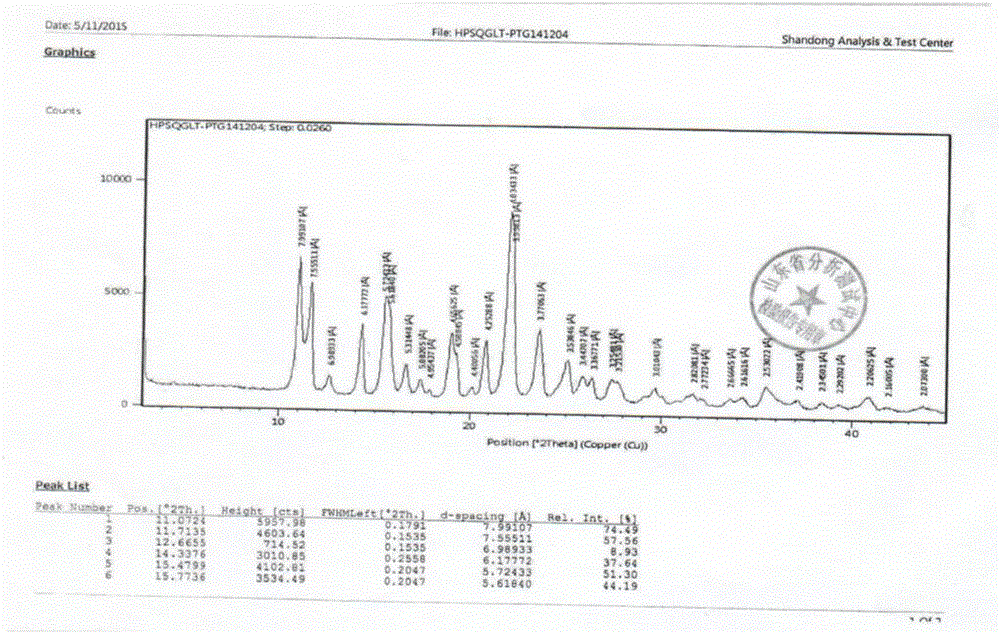
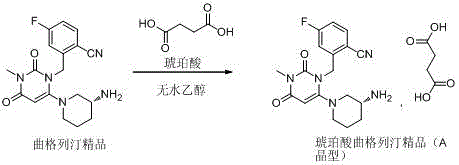
Trelagliptin succinate crystal form A preparation method
The present invention relates to a trelagliptin succinate crystal form A preparation method, and belongs to the technical field of the chemistry. The technical scheme comprises: preparing a trelagliptin fine product, and preparing a trelagliptin succinate bulk drug. The invention provides the trelagliptin succinate crystal form A preparation method suitable for industrial production, wherein the purity of the product prepared through the method is more than 99.95%; and the used single solvent anhydrous ethanol can be recovered and applied, is the optimal solvent for preparing the trelagliptin succinate crystal form A, is the bulk drug refining solvent recommended in ICH, and has characteristics of low prices and environmental protection.
Owner:WEIHAI DISU PHARMA CO LTD +1
Trelagliptin succinate rapid-release pellet preparation and preparation method thereof
The invention discloses a trelagliptin succinate rapid-release pellet preparation and a preparation method thereof, and relates to the field of pharmaceutic preparation technologies and application. The rapid-release pellet preparation is administrated orally; a core pellet is taken as a carrier, and a trelagliptin succinate aqueous solution containing a co-solvent and a bonding agent is taken as an administration solution. The trelagliptin succinate rapid-release pellet preparation has the characteristics of high administration rate, high content uniformity, rapid release, quick response of the analgesic activity and the like. Meanwhile, the rapid-release pellet preparation has high clinical use compliance, high security and the like.
Owner:FOSHAN TENGRUI MEDICINE TECH CO LTD
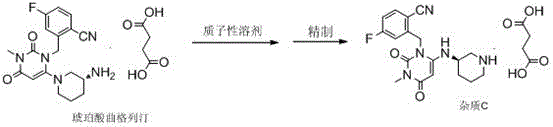
Method for preparing impurity C reference substance of Trelagliptin succinate
The invention relates to a method for preparing an impurity C reference substance of Trelagliptin succinate. The technical scheme of the invention is as follows: the method comprises the steps: (1) carrying out refluxing on Trelagliptin succinate, which serves as a raw material, and a protonic solvent which serves as a solvent, so as to prepare an impurity C of the Trelagliptin succinate; (2) refining a crude product, thereby obtaining the qualified impurity C reference substance. The method for preparing the impurity C reference substance of the Trelagliptin succinate, provided by the invention, is easy and feasible and is used for providing the satisfactory impurity C reference substance for quality control on the Trelagliptin succinate.
Owner:WEIHAI DISU PHARMA CO LTD +1
Preparation method of trelagliptin succinate
InactiveCN111349075AShort reaction timeEasy to operateCarboxylic acid salt preparationBiochemical engineeringProcess engineering
The invention provides an improved preparation method of trelagliptin succinate. The method comprises the following steps: by using 6-chloro-3-methyluracil and 2-cyano-5-fluorobenzyl bromide as initial raw materials, carrying out substitution reaction twice, refining, and carrying out a salifying reaction to obtain the trelagliptin succinate finished product. The method has the advantages of simple process, easily available raw materials, economy, environmental protection, high product yield, purity of 99% or above, and facilitation of realization of industrialization.
Owner:ZHEJIANG WAN SHENG PHARMA CO LTD
Trelagliptin succinate-related substance, preparation method, analysis method and application of substance
ActiveCN110305106ASimple manufacturing methodEasy to separateOrganic chemistryComponent separationAnalysis methodAnalytical chemistry
The invention relates to a Trelagliptin succinate-related substance L which has not been reported in literature, a preparation, analysis and detection method and an application of the substance L. Theanalysis and detection method can detect 10 related substances including related substance L, and has good separation degree and high sensitivity.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Method for refining trelagliptin succinate
The invention provides a method for refining trelagliptin succinate, wherein the method includes a dissolution step and a crystallization and separation step; the dissolution step comprises the steps of under a condition of the temperature of 30-70 DEG C, mixing a trelagliptin succinate crude product, water and an organic solvent to make the trelagliptin succinate crude product dissolved; the volume ratio of water to the organic solvent is 1 to (5-15), and the organic solvent is selected from at least one of methanol, propanol, acetone, ethanol and isopropanol. The method is used for recrystallization of the trelagliptin succinate crude product, the experimental period is short, the purification yield can be improved, and impurities also can be effectively removed; the obtained trelagliptin succinate has high purity, the purity is more than 99.9% through HPLC detection, and the product quality and the drug use safety are improved significantly.
Owner:NANJING VARSAL MEDICINE TECH DEV
Microporous membrane-coated controlled-release diacerein pellet and preparation method thereof
InactiveCN106692109APrevent excretionDoes not affect feed intakeOrganic active ingredientsAntipyreticControlled releaseAdditive ingredient
The invention discloses a microporous membrane-coated controlled-release trelagliptin succinate pellet and a preparation method thereof. The microporous membrane-coated controlled-release trelagliptin succinate pellet sequentially comprises a drug-containing pellet core and a controlled-release coating layer from inside to outside, wherein the drug-containing pellet core is prepared from a primary material and an auxiliary material at the ratio of (5%-20%):(80%-95%). According to the microporous membrane-coated controlled-release trelagliptin succinate pellet, a bitter taste can be coated, the effective ingredients can be dissolved out, constant long-acting plasma concentration is ensured, and the defect that a drug is discharged along with excrement before not completely released is avoided.
Owner:FOSHAN TENGRUI MEDICINE TECH CO LTD
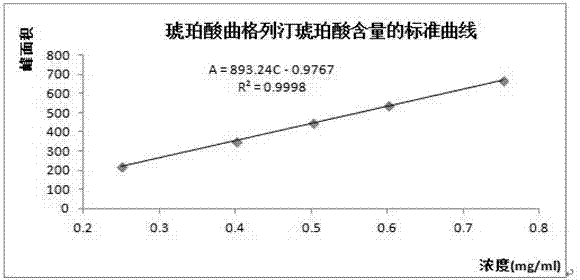
Method for measuring content of succinic acid in trelagliptin succinate
ActiveCN107884496AAccurate methodGood reproducibilityComponent separationQuality controlSuccinic acid
The invention relates to a method for measuring the content of succinic acid in trelagliptin succinate. According to the method, high performance liquid chromatography (HPLC) is adopted to measure thecontent of succinic acid in trelagliptin succinate. The method is simple and accurate, the sensitivity is high, and the repeatability is good. The method has been testified and can be applied to common analysis and quality control during the preparation process of trelagliptin succinate.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
Trelagliptin succinate solid preparation and preparation method thereof
PendingCN110063942AAvoid it happening againGuaranteed stabilityOrganic active ingredientsMetabolism disorderFluidized bed dryingAdhesive
The invention discloses a trelagliptin succinate solid preparation and a preparation method thereof. The method comprises the following steps that by mass, 50-80 parts of trelagliptin succinate, 5-20parts of a filling agent, 1-10 parts of an adhesive, 3-15 parts of a disintegrating agent and 0-5 parts of a lubricant are prepared; trelagliptin succinate, the filling agent and the adhesive are mixed proportionally, and pre-mixed powder is obtained; then, water is added into the pre-mixed powder for granulation to obtain medicated granules; the medicated granules are dried by a fluidized bed fordrying, and dried medicated granules are obtained; the disintegrating agent and the lubricant are added into the dried medicated granules, and uniform mixing is performed to obtain total mixed granules; then, pressing and tableting are performed to obtain trelagliptin tablet cores. The method is simple and feasible in technology, the dissolution of the obtained solid preparation meets the requirement of a quick release agent, the tablet wright difference is small, and stability is good.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Tablet containing trelagliptin or trelagliptin succinate and preparation method of tablet
The invention relates to a tablet containing trelagliptin or trelagliptin succinate and a preparation method of the tablet, and belongs to the technical field of medicine preparations. The tablet can be singly used or jointly used together with other oral hypoglycemic agents, and is suitable for a patient with Type II diabetics. The tablet contains the trelagliptin or the trelagliptin succinate, a thinner, an adhesive, a disintegrant and a lubricant. The preparation method of the tablet containing the trelagliptin or the trelagliptin succinate comprises the following steps of (1) obtaining of medicine particles: uniformly mixing the trelagliptin or the trelagliptin succinate, the disintegrant and / or the thinner, adding a proper amount of adhesive, and granulating, so as to obtain the medicine particles; (2) obtaining of tabletting particles: uniformly mixing the medicine particles, the lubricant, the disintegrant and / or the thinner, so as to obtain the tabletting particles; (3) compression and forming: compressing and forming the tabletting particles by proper equipment. The tablet containing the trelagliptin or the trelagliptin succinate has the advantages that the dissolvability is good, the stability is good, the technology operation is simple and convenient, and the like; the technology is simple, the repeatability is good, and the tablet is especially suitable for industrial production.
Owner:BEIJING SHENLANHAI BIO PHARM TECH
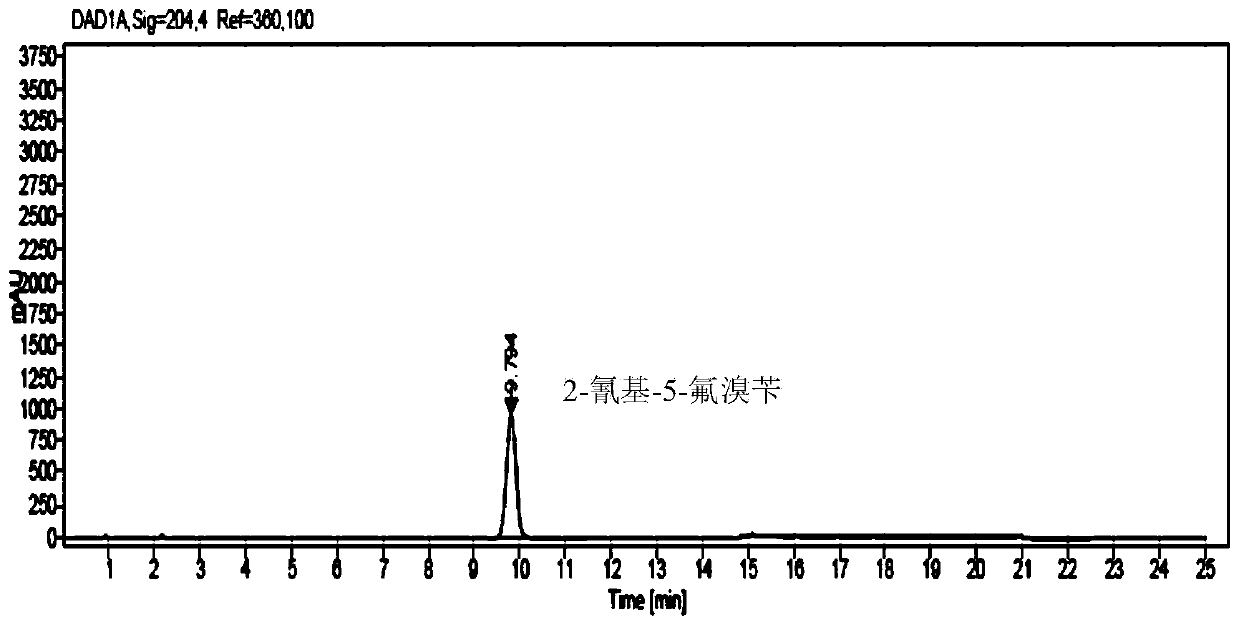
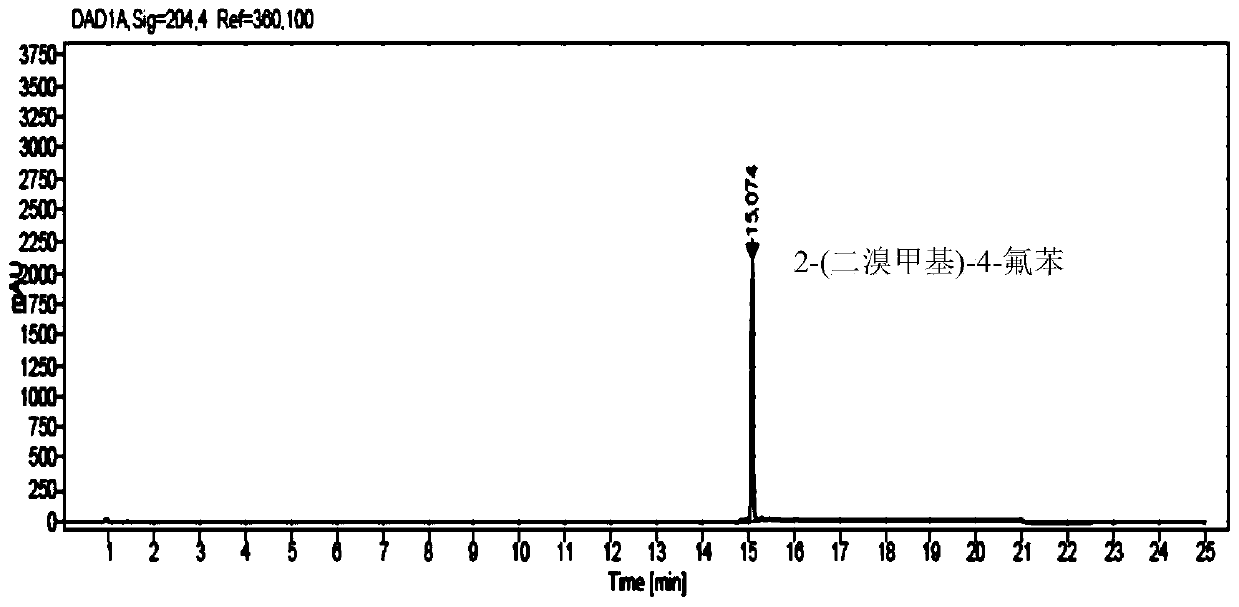
Method for analyzing and determining 2-(dibromo methyl)-4-fluorobenzonitrile in trelagliptin succinate
Owner:浙江华贝药业有限责任公司
Trelagliptin succinate tablets
InactiveCN106309393AEasy to prepareEasy to operateOrganic active ingredientsMetabolism disorderMannitolChemistry
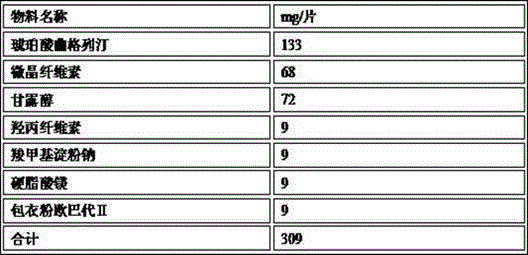
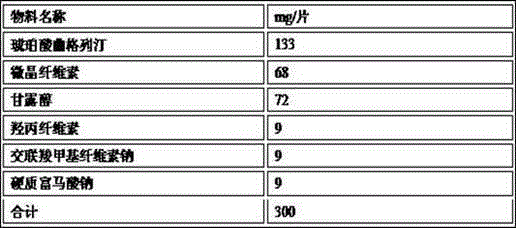
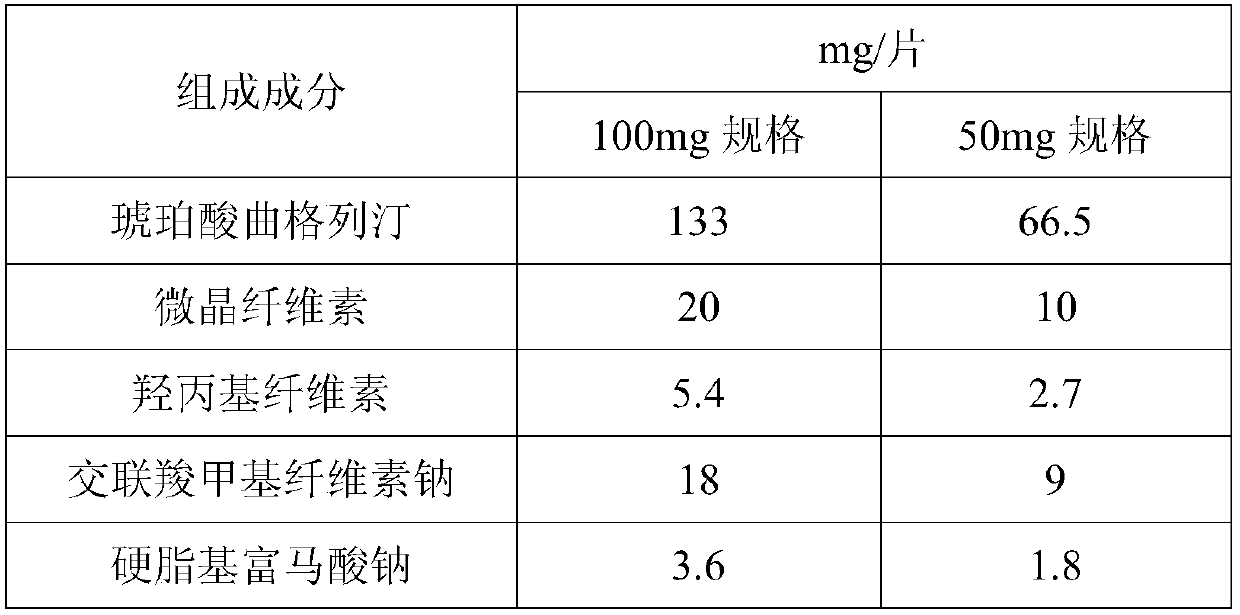
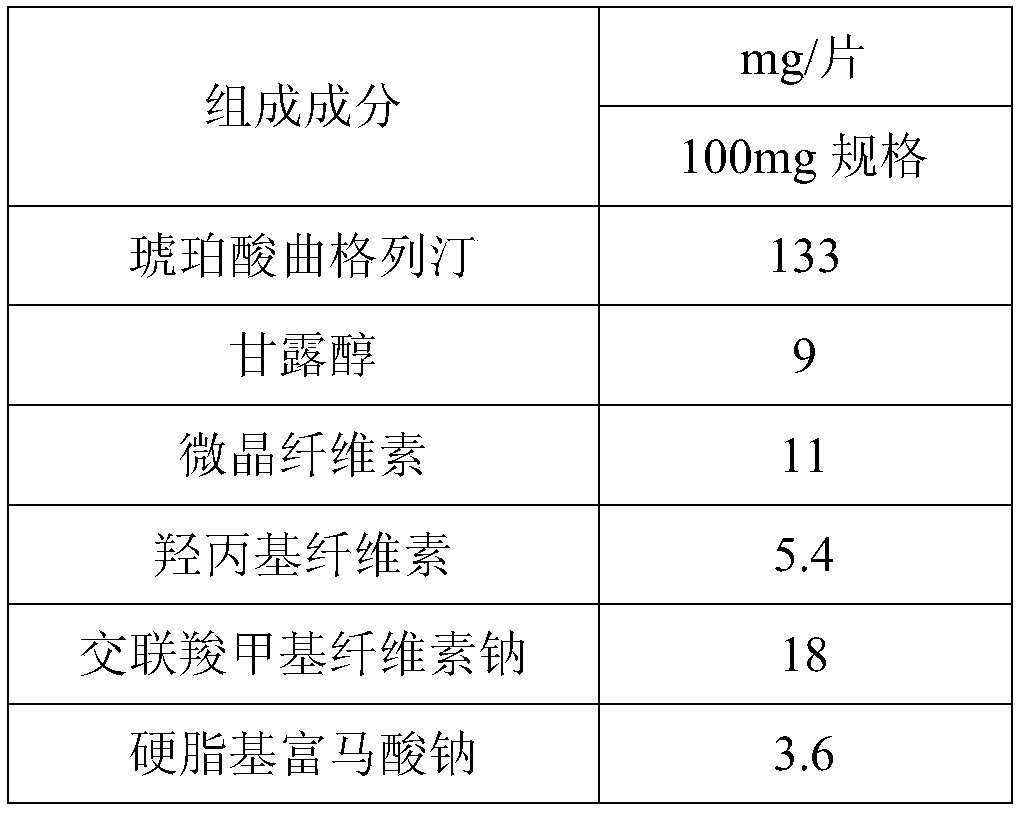
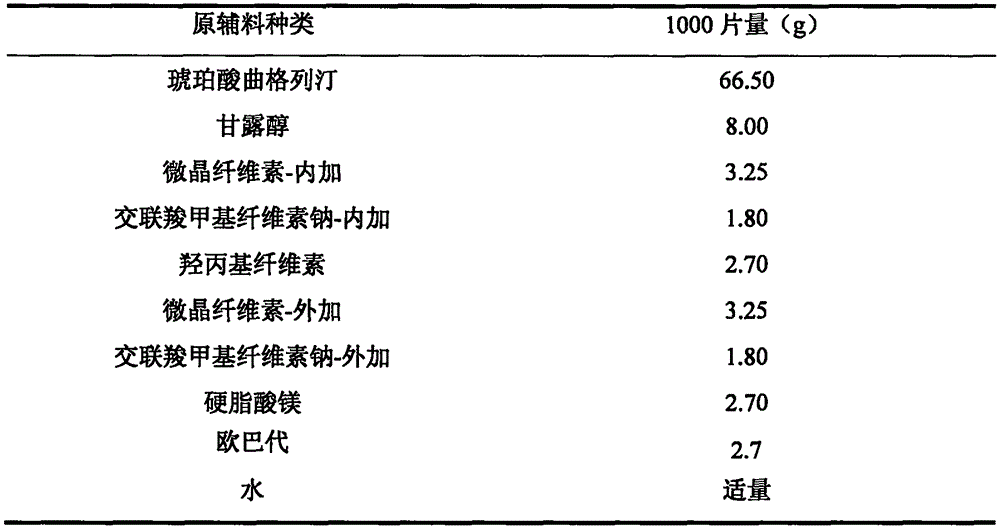
The invention provides trelagliptin succinate tablets and a preparation method thereof. The trelagliptin succinate tablets contain the following components in parts by weight: 64.0%-78.2% of trelagliptin succinate, 3.7%-5.0% of cross-linking sodium cellulose glycolate, 2.2%-5.0% of hydroxypropyl cellulose, 6.8%-25.1% of mannitol, 6.8%-15.2% of microcrystalline cellulose and 0.1%-5% of sodium stearoyl fumarate. The invention aims at providing the trelagliptin succinate tablets with even quality, high disintegration speed, good digestion degree, small tablet weight, simple production process, easy operation and suitability for industrial production.
Owner:中山万远新药研发有限公司
Purification method of trelagliptin succinate
InactiveCN106749176AEfficient removalSimple and fast operationOrganic chemistryPurification methodsSolvent
The invention relates to the technical field of medicinal chemistry and in particular relates to a purification method of trelagliptin succinate. The purification method comprises the following steps: adding a trelagliptin succinate crude product into a mixed solvent prepared from ethanol and isopropyl alcohol according to the volume ratio of (5 to 7) to 1; heating and dissolving to obtain a crude product solution; cooling and recrystallizing the crude product solution; filtering to obtain a filter cake; washing the filter cake and drying to obtain the trelagliptin succinate. The purification method provided by the invention utilizes a manner that taking the ethanol and the isopropyl alcohol according to the volume ratio of (5 to 7) to 1 as the mixed solvent for re-dissolving and recrystallizing the trelagliptin succinate, thus effectively removing impurities in the trelagliptin succinate; the yield of a trelagliptin succinate pure product obtained by the whole purification method reaches 92 percent to 95 percent and the purity reaches 99.8 percent to 99.92 percent; meanwhile, the method provided by the invention is simple and convenient to operate and is applicable to industrial popularization and application.
Owner:ZHENGZHOU MINGZE MEDICAL TECH
Preparation process of trelagliptin succinate tablet
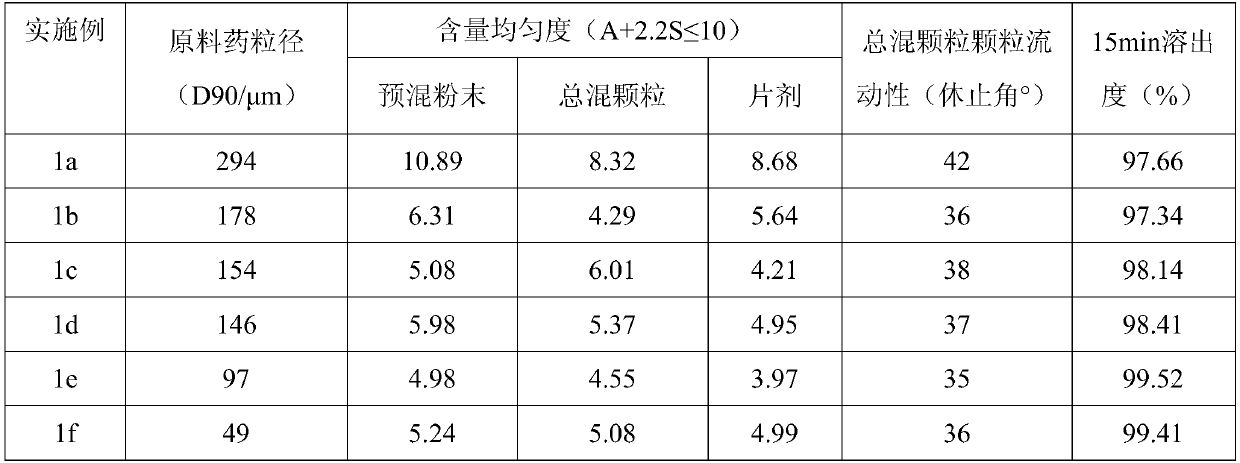
ActiveCN113975241AImprove particle propertiesWide range of designsOrganic active ingredientsPharmaceutical non-active ingredientsProcess engineeringSpray dried
The invention discloses a preparation process of a trelagliptin succinate tablet, which comprises the following steps of: mixing a carrier with an API raw material according to a preferable ratio, and granulating by using spray drying equipment under preferable parameters, so that the granule has better granule property and regular appearance property compared with an original grinding fluidized bed, and has smaller bulk volume and good flowability and compressibility compared with pure API; the risk of tablet cracking caused by difficulty in discharging air in gaps of materials in tablets and poor compressibility due to physical properties of fluidization granulation and API is avoided, so that the design range of technological parameters is wider, the technological process is more controllable and stable, various process feasibility problems caused by poor physical attributes of API, fluffy particles after fluidization granulation, poor compressibility and the like can be solved, process control is easy to realize, product quality attributes are guaranteed, meanwhile, the production process can be simplified, the production efficiency can be improved, and the production cost can be saved.
Owner:宁波高新区美诺华医药创新研究院有限公司
Method for analyzing and determining 2-cyano-5-fluorobenzyl bromide in trelagliptin succinate
The invention provides a method for analyzing and determining 2-cyano-5-fluorobenzyl bromide in trelagliptin succinate. According to the method, octadecylsilane chemically bonded silica is adopted asa stationary phase, a phosphoric acid aqueous solution and an acetonitrile solution are adopted as mobile phases, a gradient elution mode is adopted, and an established high performance liquid chromatography method can accurately determine the potential genotoxic impurity 2-cyano-5-fluorobenzyl bromide in trelagliptin succinate. The HPLC-UV method is simple to operate, short in analysis time, highin accuracy and good in repeatability; the sensitivity of the method can reach 3.0 ppm; a reliable detection method is provided for the quality standard of trelagliptin succinate; a guarantee is provided for the healthy medication of patients; and the HPLC-UV method belongs to the technical field of medicines.
Owner:浙江华贝药业有限责任公司
Preparation method of composite trelagliptin succinate tablets
ActiveCN109331019AReduce dosageSmall side effectsOrganic active ingredientsOrganic chemistrySide effectApocynum venetum
The invention provides a preparation method of composite trelagliptin succinate tablets. Preparation of trelagliptin succinate raw materials comprises the step of separately sieving 50 g-230 g of trelagliptin succinate raw materials, 300 g-1380 g of apocynum venetum plaster powder, 30 g-86 g of mannitol, 2 g-58 g of microcrystalline celluloses, 1.8 g-37 g of croscarmellose sodium and 3.2 g-54 g ofsodium stearyl fumarate by using a sieve with meshes of 60, wherein the trelagliptin succinate raw materials and the apocynum venetum plaster powder are added according to the ratio of 1: (5-7), theuse amount of drugs and the toxic and side effects of the drugs are reduced obviously, and an effect of treating diabetes is remarkable.
Owner:HONG KONG JOWA & HUAYUAN GRP CHUZHOU PHARMA CO LTD
Novel preparation process of trelagliptin succinate
InactiveCN112694465ALow costReduce usageOrganic compound preparationCarboxylic acid salt preparationTrelagliptinSuccinic acid
The invention discloses a novel preparation process of trelagliptin succinate. According to the method, 3-methyl-6-chlorouracil is taken as a starting raw material, toluene, DMF or NMP is taken as a solvent to react with 2-cyano-5-fluorobenzyl bromide under the alkaline condition to obtain 2-[(6-chloro-3, 4-dihydro-3-methyl-2, 4-dioxo-1 (2H)-pyrimidinyl) methyl]-4-fluorobenzonitrile, then the solvent system reacts with (R)-3-Boc-aminopiperidine under the alkaline condition, and the protective group is dissociated by using acid to obtain trelagliptin, and salt forming reaction is carried out on trelagliptin and succinic acid (SM4) to finally obtain the trelagliptin succinate serving as a type 2 diabetes resistant medicine. By adopting a one-pot method, the method has the advantages that the raw material cost is low, the yield is high, the post-treatment operation of each step of chemical reaction in multi-step reaction is reduced, the production period is greatly shortened, few impurities are generated in the reaction, the product quality is high, the use amount of chemical reagents is relatively reduced, and the method is relatively green and environment-friendly, and is beneficial to industrial production.
Owner:山东永丞制药有限公司
Synthesis process of trelagliptin succinate
PendingCN112939937AHigh yieldOrganic compound preparationCarboxylic acid salt preparationChemical synthesisPtru catalyst
The present invention discloses a trelagliptin succinate synthesis process, and relates to the technical field of pharmaceutical chemical synthesis. In the process, 2-cyano-5-fluorobenzyl bromide and 6-chloro-3-methyl uracil are adopted as starting raw materials, and condensation reaction is performed under the action of cuprous acetylacetonate and an acid-binding agent I to prepare an intermediate 1; then the intermediate 1 and (R)-3-aminopiperidine dihydrochloride are subjected to a condensation reaction under the action of dibutyltin dichloride and an acid-binding agent II to prepare an intermediate 2, and the intermediate 2 and succinic acid are subjected to a salt forming reaction to prepare trelagliptin succinate. Herein, the acid-binding agent and the catalyst are matched to improve the yield of the intermediate 1 and the intermediate 2, so that the total yield of the trelagliptin succinate is improved, the total yield reaches 80% or above, and the purity of the trelagliptin succinate reaches 99.5% or above.
Owner:HEFEI UNIV OF TECH
Preparation method of trelagliptin succinate dimer and application thereof
The invention belongs to the field of medicines, and particularly relates to a preparation method of a trelagliptin succinate dimer and an applicationthereof, and the method comprises the following steps: preparing a reaction raw material trelagliptin succinate; and preparing trelagliptin succinate dimer by thermally melting trelagliptin succinate under a solvent-free condition. According to the technical scheme provided by the invention, the route is simple, the reaction conditions are easy to control, the process is simple, the purity of the obtained product is relatively high, and the method can be applied to qualitative and quantitative research and detection of trelagliptin succinate related substances, and has important significance for effectively controlling the quality of trelagliptin succinate.
Owner:北京鑫开元医药科技有限公司
A kind of process improvement method for preparing Trolagliptin succinate
ActiveCN106279104BReduce pollutionOvercome the shortcomings of cumbersome industrial operationsOrganic chemistryBiochemical engineeringSuccinic acid
The invention relates to a technological improvement method for preparing trelagliptin succinate (compound V). The method comprises the following steps: by taking 6-chloro-3-methyluracil in formula (I) shown in the description and 2-cyano-5-fluorobenzyl in formula (II) shown in the description as raw materials, carrying out bromo benzyl condensation reaction to obtain a compound III, selecting an appropriate solvent and control reaction condition, enabling a midboy III to be directly subjected to piperidine condensation reaction with (R)-3-aminopiperidine dihydrochloride shown in formula (IV) without separation and purification, and finally carrying out a salifying one-pot method to prepare trelagliptin succinate (compound V). Trelagliptin succinate is prepared by using the one-pot method, so that the reaction steps are reduced, the operation process is simplified, the production efficiency is improved, and the method is safe, environmentally friendly and suitable for the industrialized production.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com