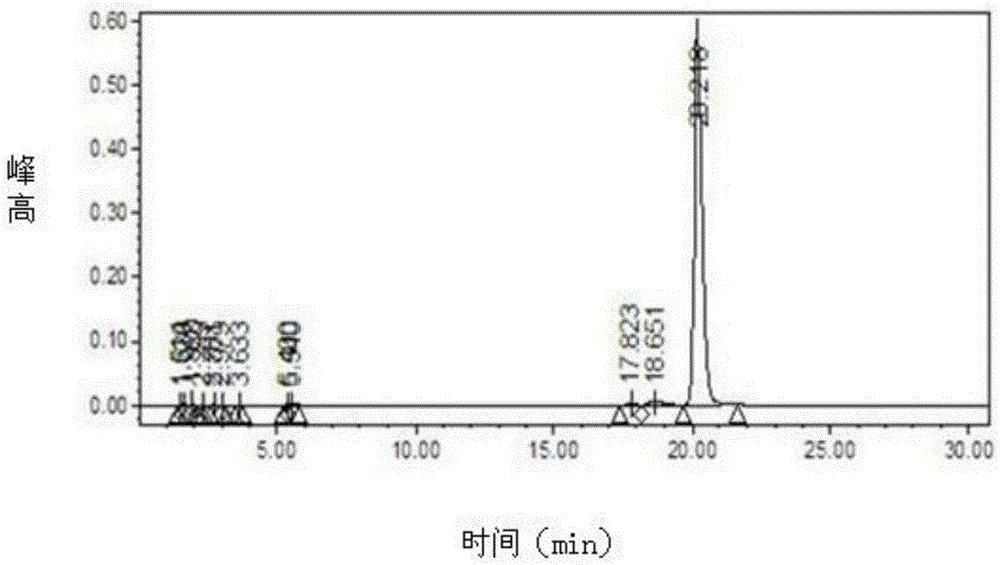
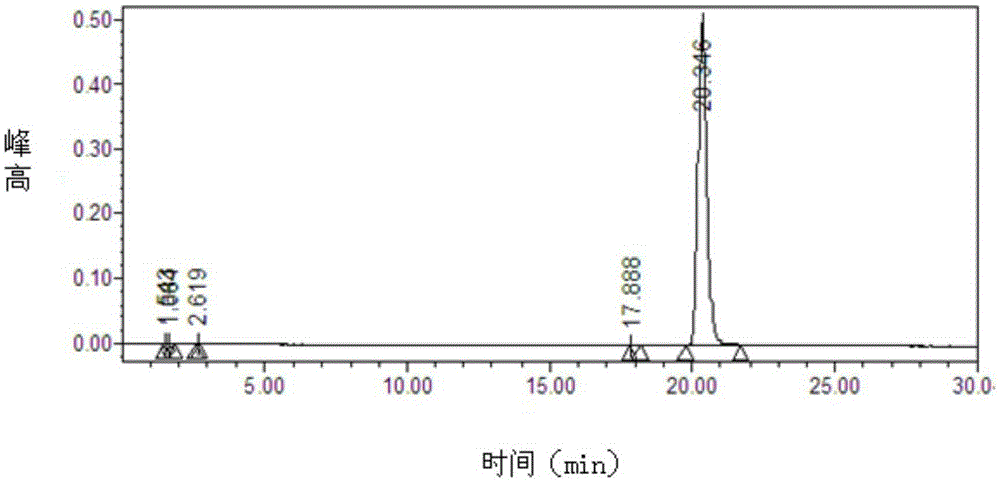
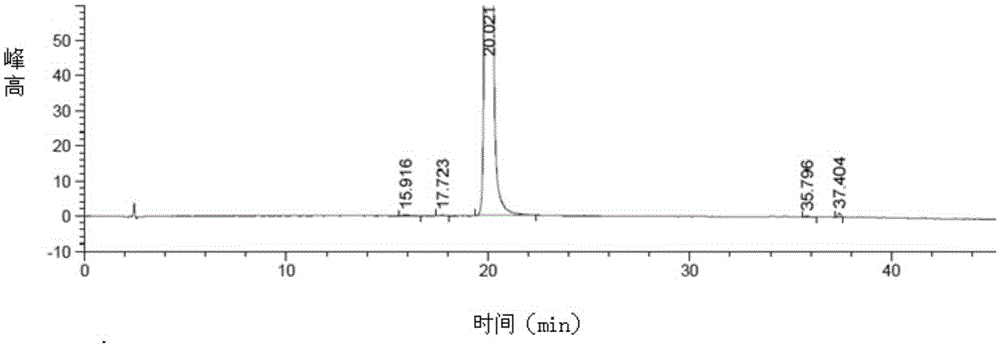
Preparation method of trelagliptin succinate
A technology of troxagliptin succinate and succinic acid, which is applied in the field of preparation of troxagliptin succinate, can solve the problems of difficulty in separating and purifying the final product, large amount of organic solvent, low yield and purity, and achieve convenient Solvent recovery, low toxicity, and short purification time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Add 29.4g (0.1mol) of formula (2) and 17.3g (0.1mol) of (R)-3-amino-piperidine dihydrochloride into a 500mL three-necked flask, then add 46.6g (0.44mol) of sodium carbonate, 1.8mL of water and 260mL of ethanol were stirred and heated to 60°C, and kept at this temperature for 6 hours. Heating was stopped and 87 mL of acetonitrile was added, stirred and cooled to room temperature. After filtering, the filter cake was washed twice with 87 mL of acetonitrile respectively, the filtrate and washing liquid were combined, and rotary evaporated to dryness under reduced pressure at 45°C to obtain 40.3 g of crude product of formula (3) as light yellow solid.
[0063] The crude product of light yellow solid formula (3) is recrystallized, and the steps are as follows:
[0064] D1: First heat 241.8ml of ethanol to reflux, add 2.9g of activated carbon after the crude product of the light yellow solid formula (3) is completely dissolved, continue heating to reflux, and filter while it...
Embodiment 2
[0069] Add 29.4g (0.1mol) of formula (2) and 19.0g (0.11mol) of (R)-3-amino-piperidine dihydrochloride into a 500mL three-necked flask, then add 51.1g (0.37mol) of potassium carbonate, 1.9 mL of water and 260 mL of isopropanol were stirred and heated to 60°C, and kept at this temperature for 5 hours to react. Heating was stopped and 87 mL of acetonitrile was added, stirred and cooled to room temperature. Filter, wash the filter cake twice with 87mL acetonitrile respectively, combine the filtrate and washing liquid, and rotary evaporate to dryness under reduced pressure at 45°C to obtain 43.2g of the crude product of the light yellow solid formula (3); the crude product of the light yellow solid formula (3) Recrystallization, the steps are as follows:
[0070] D1: First heat 238ml of ethanol to reflux, add 3.1g of activated carbon after the crude product of the light yellow solid formula (3) is completely dissolved, continue heating to reflux, and filter while it is hot;
[0...
Embodiment 3
[0074] 1063g (3.62mol) of formula (2) and 689g (3.98mol) of (R)-3-amino-piperidine dihydrochloride were added to a 20L jacketed reactor, and then potassium carbonate 2200g (15.9mol), water 64mL and 9.4L of isopropanol were stirred and heated to 60°C, and kept at this temperature for 6 hours. Heating was stopped and 3.1 L of acetonitrile was added, stirred and cooled to room temperature. After filtering, the filter cake was washed twice with 3.1 L of acetonitrile respectively, the filtrate and washings were combined, and rotary evaporated to dryness at 35°C under reduced pressure.
[0075] 6.3 L of absolute ethanol was directly added to the crude product of the obtained light yellow solid formula (3) for recrystallization, and the recrystallization method was the same as in Example 2 to obtain 1173 g of white crystals of ethanol solvate of formula (3), with a total yield of 89.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com