Trelagliptin succinate-related substance, preparation method, analysis method and application of substance
A technology for trelagliptin succinate and related substances, which is applied in the field of related substances of succinate and preparation thereof, can solve the problems of low detection and separation efficiency, long elution time, poor applicability and the like, and achieves improved safety. and controllability, the preparation method is simple and easy to implement, and the effect of improving quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: Preparation of related substance L of trexagliptin succinate
[0064]
[0065] Trexagliptin (1.0g, 2.8mmol), 2-cyano-5-fluorobenzyl bromide (0.63g, 2.9mmol), potassium carbonate (0.39g, 2.8mmol) and DMF (20mL) were added to 100mL three ports successively After the addition was completed, the system was heated to 60° C. and stirred at this temperature for 4 hours. TLC detection showed that the reaction was basically complete. Water (20 mL) was slowly added dropwise to the system at room temperature. After the dropwise addition was completed, the stirring was continued for 1 h, filtered with suction, the filter cake was washed with water and methanol respectively, and dried in vacuo to obtain 1.0 g of a white solid, which was the related substance L. Yield 73.0%, purity 99.12%.
[0066] The structure of the related substance L was confirmed, and the results are as follows:
[0067] Related Substance L: m / z:491.3[M+H] + .
[0068] 1 H-NMR (DMSO-d 6 ,...
Embodiment 2
[0069] Embodiment 2: The impact of the analysis method of related substances of trexagliptin succinate-mobile phase
[0070] The preparation of blank solvent: water: methanol=80%: 20% (volume ratio)
[0071] Preparation of mixed solution:
[0072] Weigh the appropriate amount of related substances I, Q, K, G, B, L, D, C, J, M and trexagliptin succinate respectively, add blank solvent to dissolve and quantitatively dilute to obtain about 0.5mg per 1ml solution, as a positioning solution. Measure 20 μl each of the above-mentioned related substance positioning solutions and 1 ml of the succinate trexagliptin succinate localization solution, and mix them uniformly to form a mixed solution of each related substance and succinate trexagliptin succinate.
[0073] Chromatographic conditions:
[0074] Chromatographic column: Agilent Pursuit 3C18 (4.6×150mm, 3μm)
[0075] Detection wavelength: 220nm
[0076] Column temperature: 30°C
[0077] Flow rate: 1.0ml / min
[0078] Mobile p...
Embodiment 3
[0101] Example 3: Analysis method of related substances of trexagliptin succinate-influence experiment of flow rate
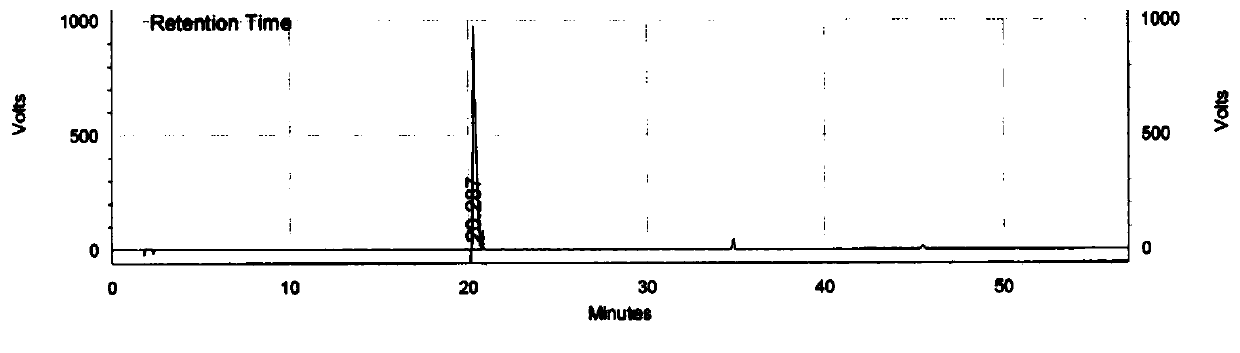
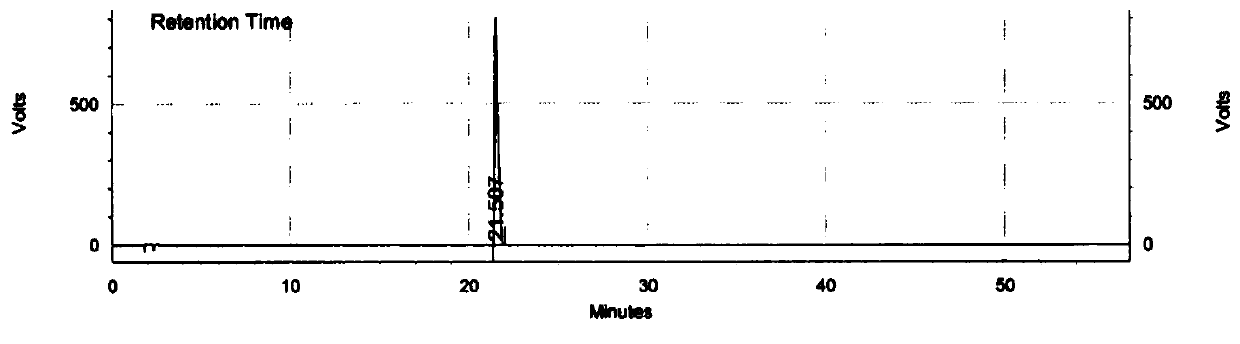
[0102] The chromatographic conditions were consistent with Experiment 3 in Example 2, only the flow rate was changed, 20 μl of the mixed solution in Example 2 was taken, injected into a liquid chromatograph, and the chromatogram was recorded, and the results of investigating the resolution of related substances were shown in Table 9 below.
[0103] Table 9 The results of investigation on the degree of separation between Trexagliptin and known related substances
[0104]
[0105] Note: " / " means no resolution, "--" means the resolution cannot be calculated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com