Preparation method for trelagliptin succinate
A technology of troxagliptin succinate and methyl, which is applied in the field of preparation of troxagliptin succinate, can solve problems such as difficult removal of residual solvents, unfavorable industrial production, cumbersome post-processing, etc., and achieves simple and easy production process, Improved purity and yield, and easy post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation method of trexagliptin succinate comprises the following steps:
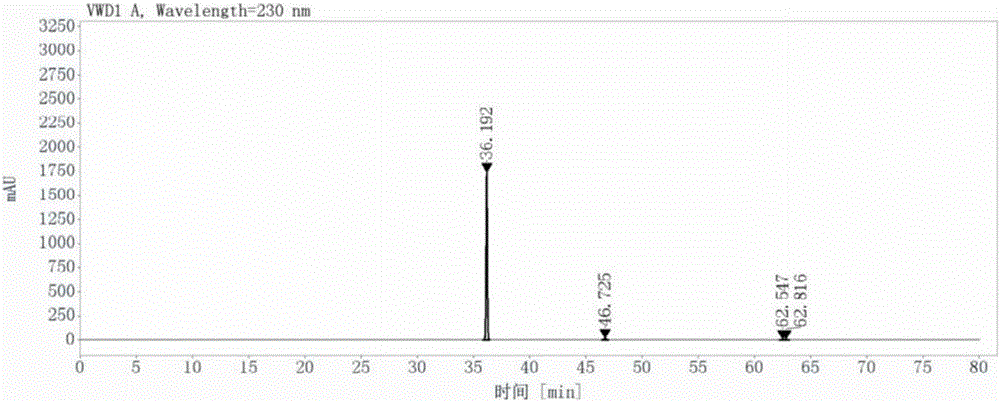
[0050] (1) Add 100ml of N-methylpyrrolidone, 25g of 3-methyl-6-chlorouracil, and 21.9g of N,N-diisopropylethylamine in turn to the reaction flask, then stir and dissolve at room temperature; dissolve Afterwards, 2-cyano-5-fluorobenzyl bromide solution was added dropwise to the above mixture at 20°C; after the dropwise addition, the system was heated to 55°C, and the reaction was stopped after 3 hours of reaction; 150ml of Purified water, stirred at -15°C for 1 hour, suction filtered, washed with ethyl acetate three times after the suction filtration, the amount of ethyl acetate each time was 30ml, and then dried in a vacuum oven at 40°C until constant Heavy, obtain 2-[(6-chloro-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H) base)methyl]-4-fluorobenzonitrile 44.8g, The yield was 98.0%, and its purity was 98.9% as detected by liquid chromatography, as shown in Figure 1-1 and Figure 1-2;
[...
Embodiment 2
[0059] The preparation method of trexagliptin succinate comprises the following steps:
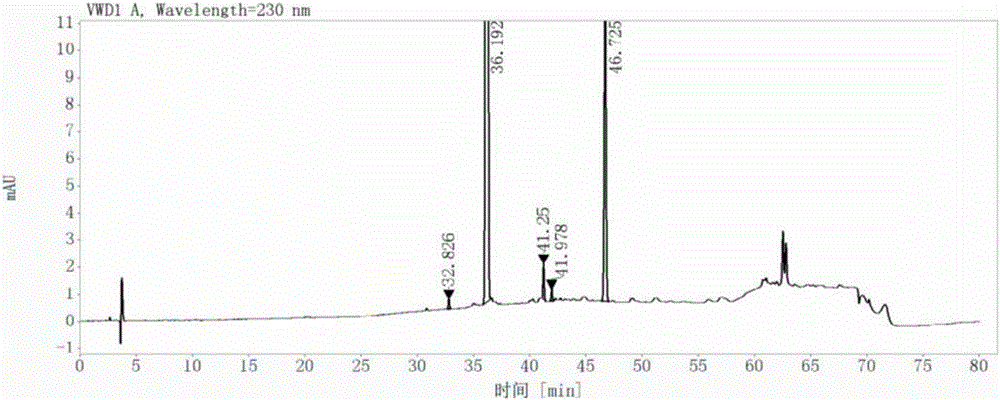
[0060] (1) Add 280ml of N-methylpyrrolidone, 75g of 3-methyl-6-chlorouracil, and 65.7g of N,N-diisopropylethylamine in turn to the reaction flask, then stir and dissolve at room temperature; Afterwards, 2-cyano-5-fluorobenzyl bromide solution was added dropwise to the above mixture at 40°C; after the dropwise addition, the system was heated to 55°C, and the reaction was stopped after 4 hours of reaction; 480ml of Pure water, stirred at 0°C for 1 hour, suction filtered, washed with isopropanol 3 times with 90ml each time after suction filtration, and then dried to constant weight in a vacuum oven at 40°C to obtain 2- [(6-Chloro-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)yl)methyl]-4-fluorobenzonitrile 135.7g, yield 98.9%, Detected by a liquid chromatograph, its purity is 98.9%, as shown in Figure 4-1 and Figure 4-2;
[0061] Wherein said 2-cyano-5-fluorobenzyl bromide solution is a solu...
Embodiment 3
[0069] The preparation method of trexagliptin succinate comprises the following steps:
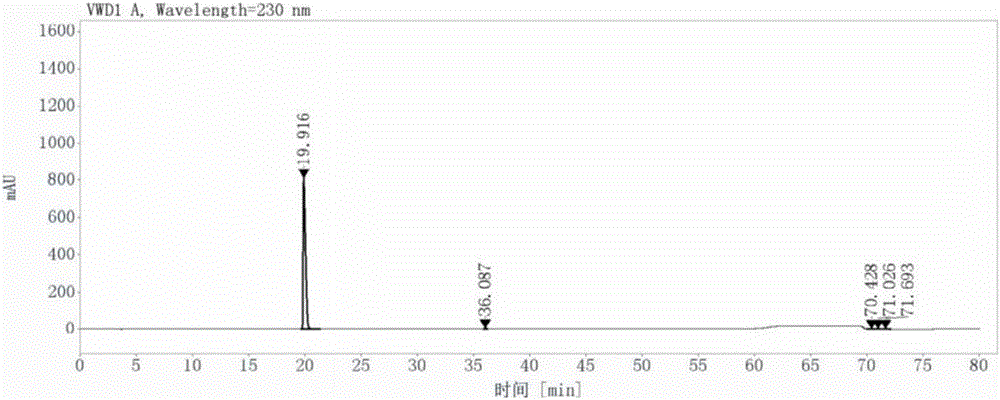
[0070] (1) Add 220ml of N-methylpyrrolidone, 50g of 3-methyl-6-chlorouracil, and 45.6g of N,N-diisopropylethylamine in turn to the reaction flask, then stir and dissolve at room temperature; After that, add 2-cyano-5-fluorobenzyl bromide solution dropwise to the above mixture at 35°C; after the dropwise addition, raise the temperature of the system to 65°C, stop the reaction after 3.5 hours of reaction; add 300ml of purified water, stirred at 35°C for 1 hour, suction filtered, washed with isopropanol after suction filtration, and then dried to constant weight in a vacuum oven at 30°C to obtain 2-[(6-chloro-3 -Methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)yl)methyl]-4-fluorobenzonitrile 89.6g, the yield was 98.0%, carried out by liquid chromatography Its purity of 98.5% was detected, and the results are shown in Figure 7-1 and Figure 7-2;
[0071] Wherein said 2-cyano-5-fluorobenzyl bromide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com