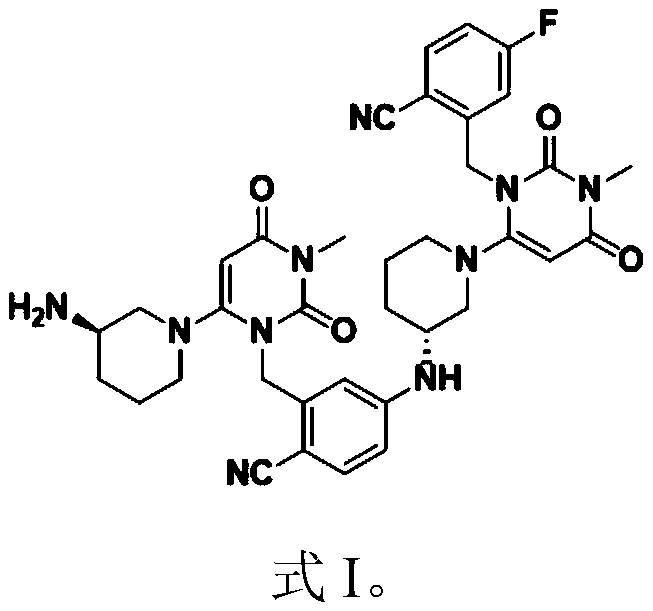
Preparation method of trelagliptin succinate dimer and application thereof
A technology of troxagliptin succinate and dimer, which is applied in the field of medicine, can solve the problems such as difficult removal of dimer impurities, and achieve the effects of quality control, simple route, and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] In a first aspect, the embodiment of the present invention provides a method for preparing a trilagliptin succinate dimer, comprising the following steps:
[0015]
[0016] Step S10, prepare the reaction raw material troglitatin succinate;
[0017] In step S20, under solvent-free conditions, the trilagliptin succinate is thermally melted to prepare a trilagliptin succinate dimer.
[0018] In the preparation method of the trilagliptin succinate dimer provided in this example, the trilagliptin succinate is used as the raw material, and the prepared trilagliptin succinate can be directly melted under solvent-free conditions. The reaction proceeds to produce a dimer of trilagliptin succinate. The preparation method has the advantages of simple route, easily controllable reaction conditions, simple process and high purity of the obtained product, and can be applied to the qualitative and quantitative research and detection of related substances of trilagliptin succinate....
Embodiment 1
[0034] In step S1, 50 g (0.105 mol) of the reaction raw material, troxagliptin succinate, was prepared.
[0035] Step S2, add the prepared trilagliptin succinate to the single-neck bottle, stir, heat up to 240° C., heat-melt the trilagliptin succinate, the reaction time is 1h, and the thin-layer chromatography monitoring shows that the trilagliptin succinate is monitored. When less than 20% of tin remained, the reaction was stopped, the reactant was dissolved in dichloromethane, and then concentrated to obtain a yellowish oily substance.
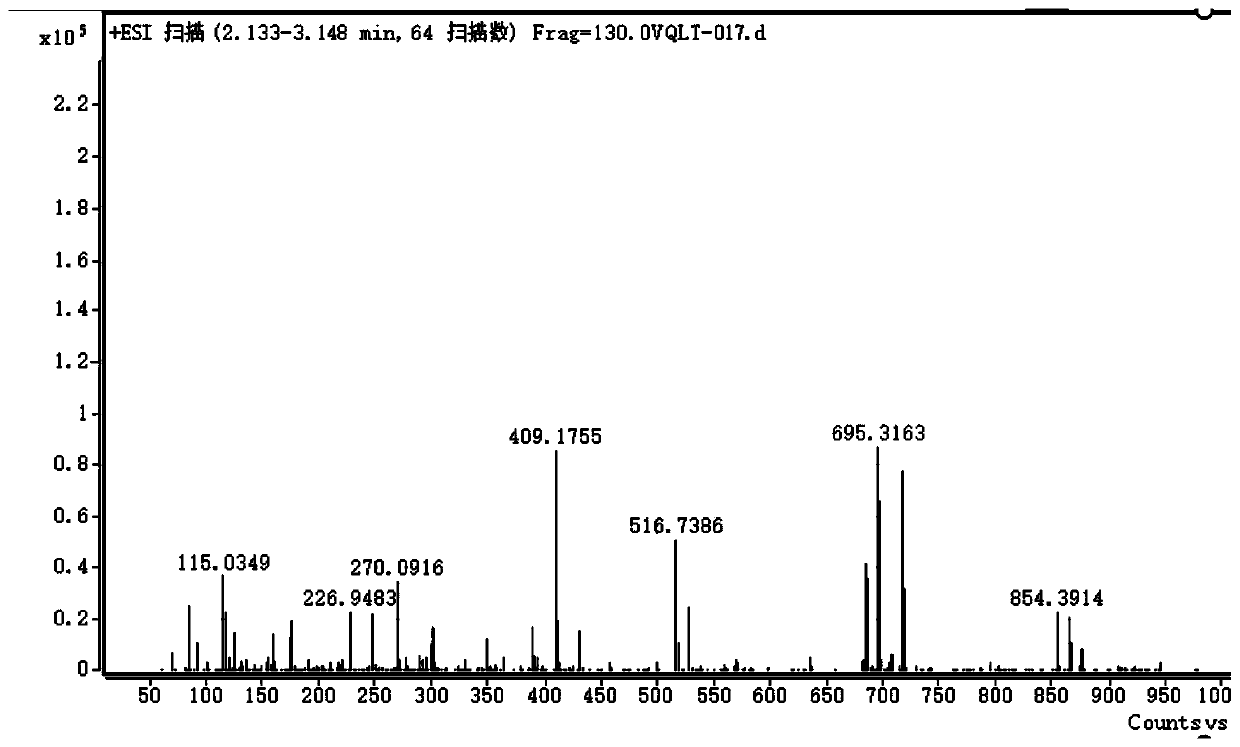
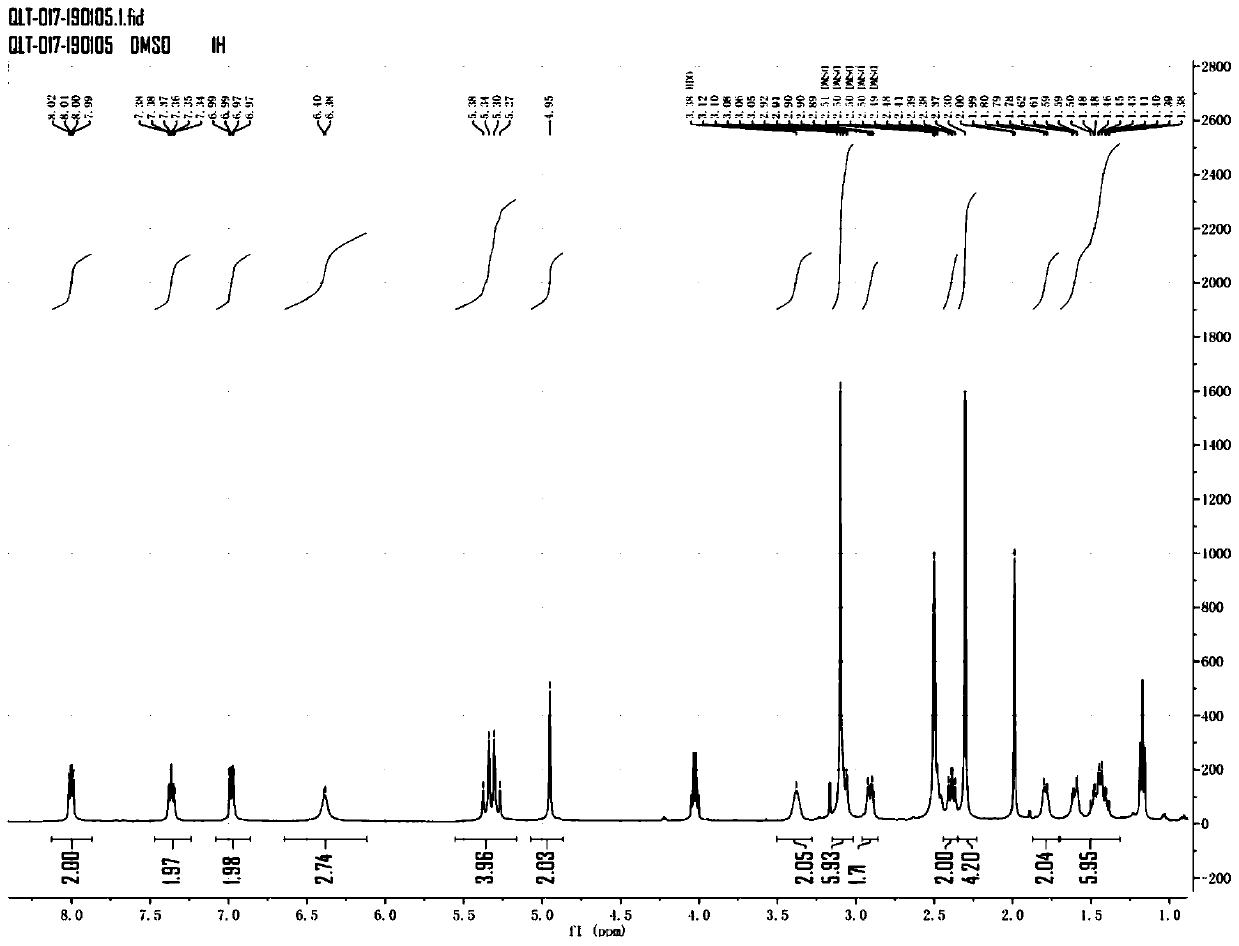
[0036] Mass spectrometry detection of the yellow oily substance was obtained. figure 1 The mass spectrum shown in the figure, the NMR detection of the yellow oily substance was obtained. figure 2 NMR spectrum shown. in figure 2 The results of the NMR spectrum shown are as follows:
[0037] Chemical shift (ppm) hydrogen number 1.38.14~1.622 6 1.6732~1.80 2 2.301.56~2.38 4 2.39~2.43 2 2.70~2.80 2 ...
Embodiment 2
[0043] In step S1, 50 g (0.105 mol) of the reaction raw material, troxagliptin succinate, was prepared.
[0044] Step S2, add the prepared trilagliptin succinate into the single-neck bottle, stir, heat up to 220° C., heat-melt the trilagliptin succinate, the reaction time is 0.5h, and the thin-layer chromatography monitoring shows that the troxagliptin succinate is monitored. When less than 20% of the liptin remained, the reaction was stopped, the reactant was dissolved in dichloromethane, and then concentrated to obtain a crude product of the dimer of trilagliptin succinate. The content measured by high performance liquid chromatography was 84.5%, and the yield was 55.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com