Trelagliptin succinate solid preparation and preparation method thereof
A technology of troxagliptin succinate and solid preparations, which is applied in the field of solid preparations of troxagliptin succinate and its preparation, can solve the problems of product content uniformity and content not meeting quality standards, unstable dry granulation process, and Problems such as poor rolling formability of auxiliary materials have achieved the effects of good production process reproducibility, stable and controllable process parameters, and improved sticking and astringent punching problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
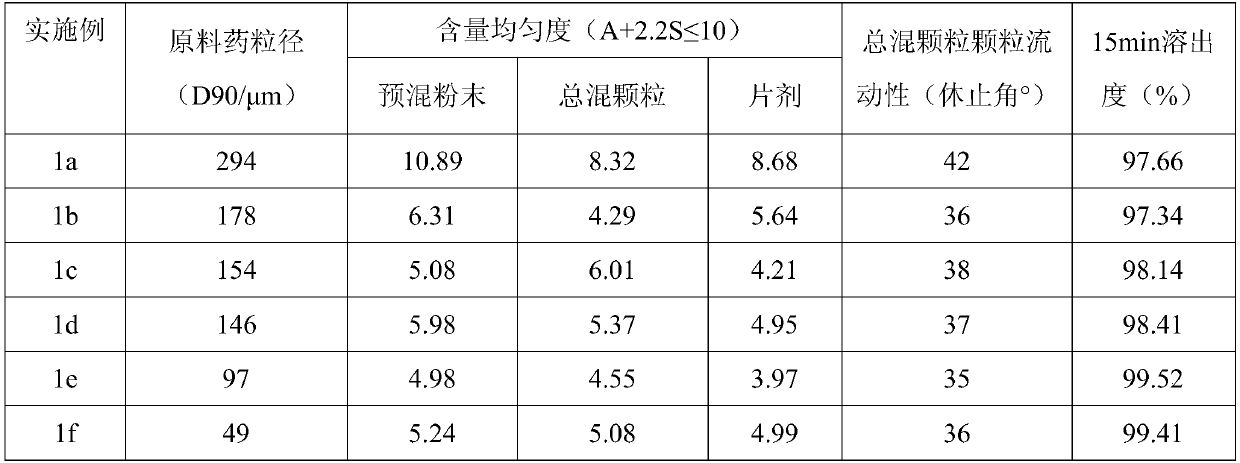
[0034] Inspection of the particle size of the raw material drug: the raw material drug is processed in different ways, and the particle size of the raw material drug (D 90 ) are 294 μm, 178 μm, 154 μm, 146 μm, 97 μm, and 49 μm, respectively. Raw materials with different particle sizes were taken to investigate the effect of particle size on the tablet preparation process and tablet quality.
[0035] D. 90 (Also known as D(0.9)) indicates the particle size corresponding to when the cumulative particle size distribution number of a sample reaches 90%. Its physical meaning is that the particles with a particle size smaller than it account for 90%.
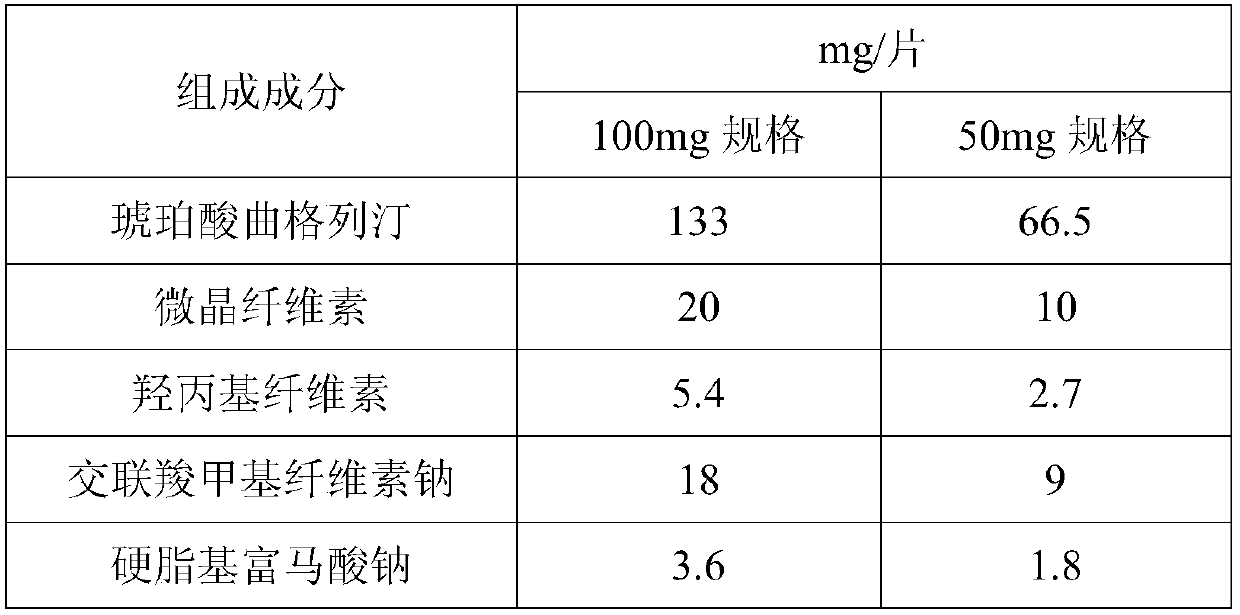
[0036] Table 1: Embodiment 1 prescription composition
[0037]
[0038] Preparation Process:
[0039] ① API pretreatment: Take an appropriate amount of trexagliptin succinate API (API) and perform the following pretreatments: untreated, passed through a 40-mesh sieve, passed through a 60-mesh sieve, passed through a 80-mesh siev...
Embodiment 2
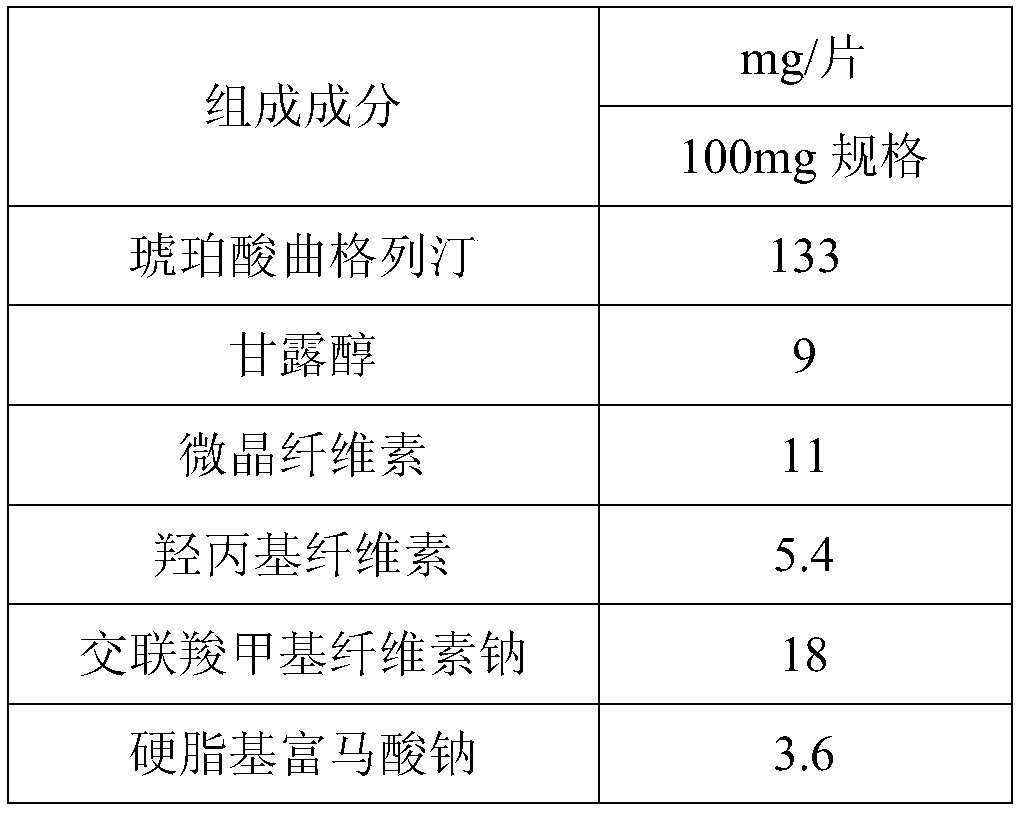
[0080] Table 4: Embodiment 2a and 2b prescription composition
[0081]
[0082] Preparation Process:
[0083] ① Weigh API, microcrystalline cellulose, and hydroxypropyl cellulose according to the prescription amount, and mix them evenly to obtain premixed powder.
[0084] ② Take an appropriate amount of purified water, add it to the premixed powder, prepare soft materials with a high-efficiency wet granulator, and perform wet granulation with a granulator to obtain drug-containing granules.
[0085] ③ Place the drug-containing granules in a fluidized bed for drying, and use a granulator to perform dry sizing to obtain the dried drug-containing granules.
[0086] ④ Take the prescription amount of croscarmellose sodium and magnesium stearate or stearic acid, add it to the dried drug-containing granules and mix evenly to obtain the total blend granules.
[0087] ⑤Using tablet pressing equipment and molds to prepare trexagliptin tablet cores, and coating the tablet cores with...
Embodiment 3
[0089] Table 5: Embodiment 3a and 3b prescription composition
[0090]
[0091] Preparation Process:
[0092] ① Weigh the API, lactose or pregelatinized starch, and hydroxypropyl cellulose according to the prescription amount, and mix them evenly to obtain the premixed powder.
[0093] ② Take an appropriate amount of purified water, add it to the premixed powder, prepare soft materials with a high-efficiency wet granulator, and perform wet granulation with a granulator to obtain drug-containing granules.
[0094] ③ Place the drug-containing granules in a fluidized bed for drying, and use a granulator to perform dry sizing to obtain the dried drug-containing granules.
[0095] ④ Take the prescription amount of croscarmellose sodium and sodium stearyl fumarate, add them to the dried drug-containing granules and mix evenly to obtain the total mixed granules.
[0096] ⑤Using tablet pressing equipment and molds to prepare trexagliptin tablet cores, and coating the tablet cores...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com