Tablet containing trelagliptin or trelagliptin succinate and preparation method of tablet
A technology of trelagliptin succinate and tableting, which is applied to the preparation field of tablets containing trelagliptin or its succinate, and can solve the problem of poor compressibility and enlargement of tablets. It can solve the problems of dosage size, inconvenience of granulation, etc., to achieve the effect of good dissolution, simple process operation and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: preparation embodiment
[0020]
[0021]
[0022] Preparation Process:
[0023] (1) prepare hydroxypropyl methylcellulose aqueous solution to make binder, for subsequent use;
[0024] (2) Trexagliptin succinate crude drug is pulverized through an 80 mesh sieve;
[0025] (3) The bulk drug is mixed with mannitol, microcrystalline cellulose and croscarmellose sodium in equal amounts;
[0026] (4) Add adhesive to make soft material by wet method, stir quickly, shear quickly, granulate with 20 mesh, dry, and granulate with 30 mesh;
[0027] (5) Add sodium stearyl fumarate and microcrystalline cellulose to the above mixture, mix well and press into tablets with a tablet weight of 180mg and a hardness of 7-9kg. Opadry coating, weight gain of about 3.0%
Embodiment 2
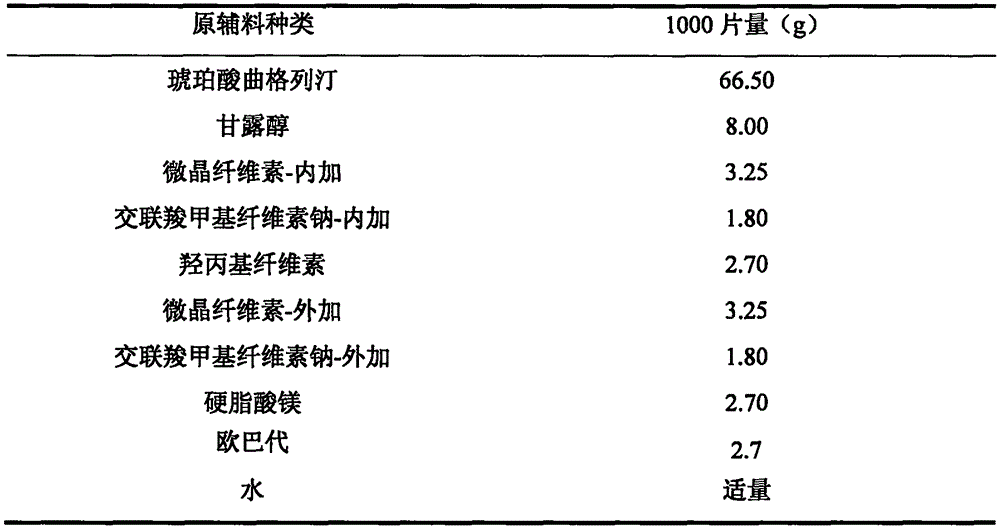
[0028] Embodiment 2: preparation embodiment
[0029]
[0030] Preparation Process:
[0031] (1) prepare the aqueous solution of hydroxypropyl cellulose to make binder, for subsequent use;
[0032] (2) Trexagliptin succinate crude drug is pulverized through an 80 mesh sieve:
[0033] (3) The bulk drug is mixed with mannitol, microcrystalline cellulose-internal addition and croscarmellose sodium-internal addition in equal amounts;
[0034] (4) Add adhesive to make soft material by wet process, stir quickly, shear quickly, granulate with 20 mesh, dry, and granulate with 24 mesh;
[0035] (5) Add microcrystalline cellulose-extra, croscarmellose sodium-extra and lubricant to the above mixture, mix well and press into tablets with a tablet weight of 90 mg and a hardness of 5-7 kg. Opadry coated, approximately 3.0% weight gain.
Embodiment 3
[0036] Embodiment 3: preparation embodiment
[0037]
[0038] Preparation Process:
[0039] (1) prepare the aqueous solution of hydroxypropyl cellulose to make binder, for subsequent use;
[0040] (2) Trexagliptin succinate crude drug is pulverized through an 80 mesh sieve;
[0041] (3) The bulk drug is mixed with mannitol, microcrystalline cellulose-internal addition and croscarmellose sodium in equal amounts;
[0042] (4) Add adhesive to make soft material by wet process, stir quickly, shear quickly, granulate with 20 mesh, dry, and granulate with 24 mesh;
[0043] (5) Add microcrystalline cellulose-additive and lubricant to the above mixture, mix well and press into tablets with a tablet weight of 180 mg and a hardness of 7-9 kg. Opadry coated, approximately 3.0% weight gain.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com