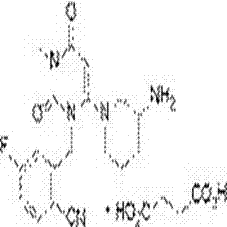
Application of asymmetric hydrogenation in synthesis of Trelagliptin intermediate
An intermediate and asymmetric technology, applied in the field of drug synthesis, can solve problems such as low product yield, high cost, and complexity, and achieve the effect of simple synthesis method, good product quality, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0028] Preparation of compound (3)
[0029] Add 20 L of absolute ethanol and 2 kg (10 mol) of compound (2) as the reaction solvent into a 50 L reactor at room temperature, then add 848 g (11 mol) of ammonium acetate, and heat the reaction solution to reflux after the addition. The reaction was tracked by TLC. After 7 hours, the reaction was completed. The temperature was lowered to about 25°C, 20L of water was added, stirred and crystallized for 2 hours, filtered, the filter cake was washed with 10L of water, and dried to obtain 1.86 kg (9.37 mol) of compound (3). The rate is 93.7%. HPLC detection purity: 97.7%.
[0030] 1 H NMR (400 MHz, DMSO- d 6 ) δ 6.92 (s, 2H), 5.97 – 5.70 (m, 1H), 3.10 –2.83 (m, 2H), 2.05 – 1.80 (m, 4H), 1.43 (s, 9H).
[0031] ESI+ [M-Boc] + =97.
[0032] Preparation of compound (4)
[0033] Add 1.78 kg (9 mol) of compound (3) in 25L of anhydrous dichloromethane to a 50L autoclave at room temperature, add [Rh(cod) 2 ] BF 4 36 g (0.09 mol) and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com