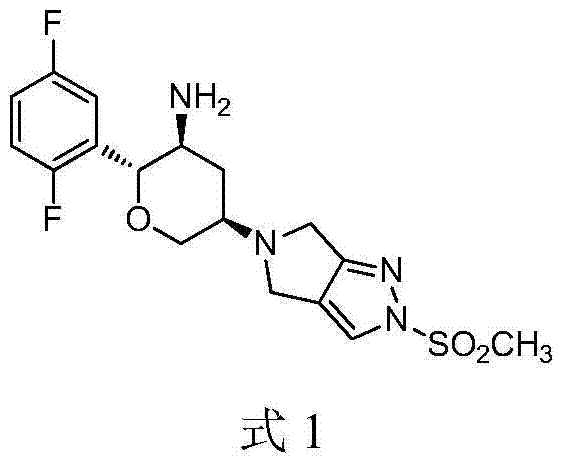
A kind of synthetic method of alogliptin
A synthetic method and a series of technologies, applied in the field of drug synthesis, can solve the problems of low product yield, high cost, and poor quality, and achieve the effect of simple synthesis method, good product quality, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
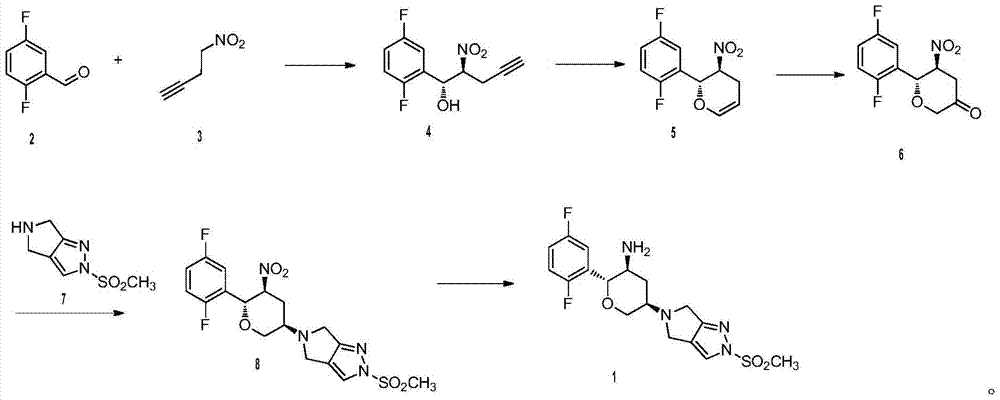
[0024] Preparation of compound (4)
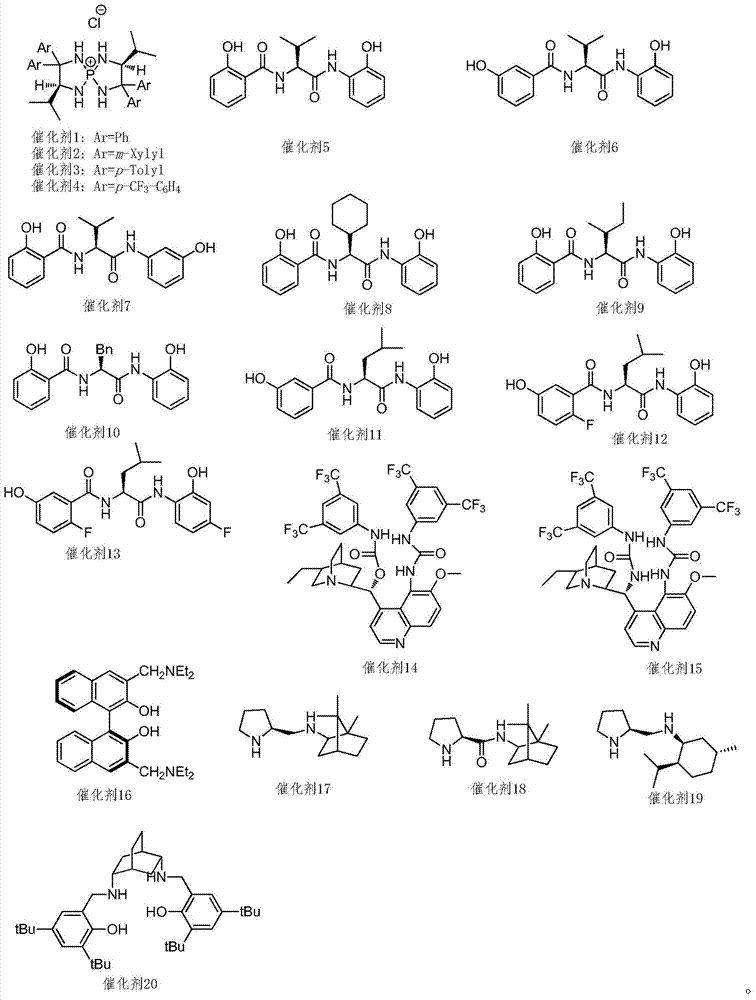
[0025] Under nitrogen protection, add 1.2kg (12mol) compound (3) and 31g (0.05mol) catalyst 3 in 20L anhydrous tetrahydrofuran in 50L reactor at room temperature, slowly drop 1.42kg (10mol) compound (2 ). Slowly add 1.94kg (15mol) of diisopropylethylamine after stirring for half an hour, after TLC monitors that the reaction is completed, the reaction solution is neutralized to pH 6.8 with acetic acid, filtered, the filter cake is washed with 5L tetrahydrofuran, and the filtrate and washings are combined. The crude product of compound (4) was obtained by concentration, and the crude product was recrystallized twice from ethyl acetate to obtain 2.13 kg (8.83 mol) of the refined product of compound (4), with a yield of 88.3%. Purity by HPLC: 98.2%.
[0026] 1 H NMR (400MHz, DMSO-d 6 )δ7.25-7.15(m,1H),7.13-7.06(m,2H),7.40-5.27(m,2H),2.97-2.87(m,1H),2.93(s,1H),2.72-2.62( m,1H).
[0027] ESI+[M+H] + =242.
[0028] Preparation of compound ...
Embodiment 2
[0045] Preparation of compound (4)
[0046] According to the method of Example 1, the reaction solvent was replaced by anhydrous toluene; the reaction temperature was -78°C, and the molar ratio of compound (2) and compound (3) was 1:1; the base used in the reaction was replaced by NaHMDS. The reaction catalyst is catalyst 3; the molar ratio of compound (2) to the catalyst is 200:1, and finally the refined product of compound (4) is obtained with a yield of 87.4%. Purity by HPLC: 97.8%.
[0047] Preparation of compound (5)
[0048] According to the method of Example 1, the solvent is replaced by anhydrous DMA, the reaction temperature is 20-80°C, and the preferred temperature is 65-75°C, the base is replaced by sodium bicarbonate, and the catalyst is replaced by bis(triphenylphosphine) dichloride Palladium, the molar ratio of compound (4) to the catalyst is 100:1; the ligand is replaced by PipPhos, and finally the refined product of compound (5) is obtained with a yield of 92...
Embodiment 3
[0056] Preparation of compound (4)
[0057] According to the method of Example 1, the reaction solvent is replaced by anhydrous dichloromethane; the reaction temperature is 110 ° C, and the molar ratio of compound (2) and compound (3) is 1:2.0; the base used in the reaction is replaced by tert-butyl Potassium alcohol. The reaction catalyst is catalyst 5; the molar ratio of the compound (2) to the catalyst is 200:1-20, and finally the refined product of the compound (4) is obtained with a yield of 89.3%. Purity by HPLC: 98.8%.
[0058] Preparation of compound (5)
[0059] According to the method of Example 1, the solvent is replaced by anhydrous DMF, the reaction temperature is 80 ° C, the base is replaced by triethylamine, and the catalyst is replaced by [(3-F-Ph) 3 P] 3 RhCl, the molar ratio of compound (4) to catalyst was 100:10; the ligand was replaced by BINAP, and finally the refined product of compound (5) was obtained with a yield of 93.6%. Purity by HPLC: 97.9%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com