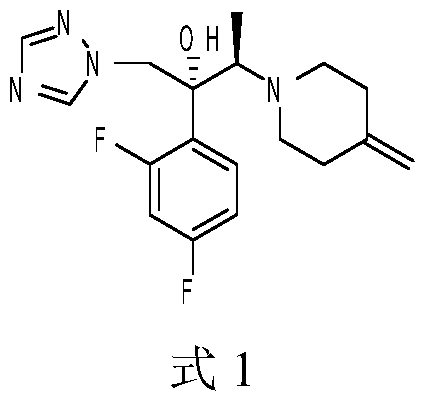
A kind of synthetic method of efluconazole intermediate
A technology of efluconazole and a synthesis method, which is applied in the field of pharmaceutical intermediate synthesis, can solve the problems of numerous intermediates, complicated processes, and many synthesis steps, and achieves the effects of high yield, good product quality and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
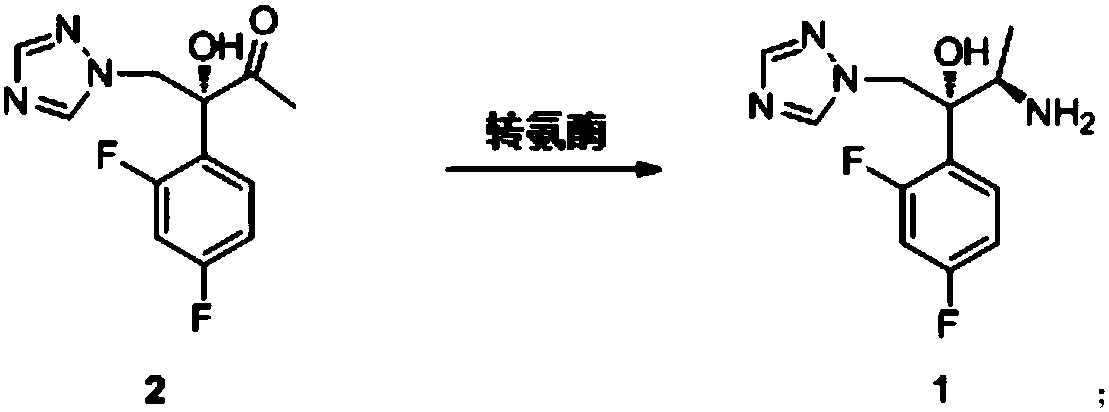
[0029] At 25°C, add the reaction solvent to the reaction kettle, then add the ammonia source, adjust the pH to 8.0 with hydrochloric acid, then add PLP and transaminase H62T, stir slowly until all are dissolved, then add compound (2) and react for 16 hours, After the reaction, adjust the pH to 2.0 with hydrochloric acid, add isopropyl acetate for extraction, leave the aqueous phase, adjust the pH to 12.0 with aqueous sodium hydroxide solution, add isopropyl acetate for extraction, and concentrate the isopropyl acetate under reduced pressure to obtain the compound ( 1).
[0030] The reaction solvent is water and dimethylsulfoxide, and the volume ratio of the two is 1:1.
[0031] The ammonia source is isopropylamine and triethylamine, the concentration is 0.5M, and the molar ratio of the two is 1:1.
[0032] The mass concentration of the compound (2) is 100g / L.
[0033] The mass concentration ratio of the compound (2) and the directed mutant of transaminase ATA-117 is 35:1.
...
Embodiment 2
[0036] At 22°C, add the reaction solvent to the reaction kettle, then add the ammonia source, adjust the pH to 7.5 with hydrochloric acid, then add PLP and transaminase G69C, stir slowly until all are dissolved, then add compound (2) and react for 14 hours. After the reaction, adjust the pH to 2.0 with hydrochloric acid, add isopropyl acetate for extraction, leave the aqueous phase, adjust the pH to 12.0 with aqueous sodium hydroxide solution, add isopropyl acetate for extraction, and concentrate the isopropyl acetate under reduced pressure to obtain the compound ( 1).
[0037] The reaction solvent is selected from water / methanol.
[0038] The source of ammonia is isopropylamine with a concentration of 0.2M.
[0039] The mass concentration of the compound (2) is 2g / L.
[0040] The mass concentration ratio of the compound (2) and the directed mutant of transaminase ATA-117 is 1:1.
[0041] The obtained intermediate was detected according to the aforementioned method, and its...
Embodiment 3
[0043] At 45°C, add the reaction solvent to the reaction kettle, then add the ammonia source, adjust the pH to 8.5 with hydrochloric acid, then add PLP and transaminase A209L, stir slowly until all are dissolved, then add compound (2) and react for 18 hours, After the reaction, adjust the pH to 2.0 with hydrochloric acid, add isopropyl acetate for extraction, leave the aqueous phase, adjust the pH to 12.0 with aqueous sodium hydroxide solution, add isopropyl acetate for extraction, and concentrate the isopropyl acetate under reduced pressure to obtain the compound ( 1).
[0044] The reaction solvent is selected from water / dimethylsulfoxide / methanol, and the volume ratio of the three is 1:1:1.
[0045] The source of ammonia is butylamine at a concentration of 1M.
[0046] The mass concentration of the compound (2) is 200g / L.
[0047] The mass concentration ratio of the compound (2) and the directed mutant of transaminase ATA-117 is 70:1.
[0048] The obtained intermediate wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com