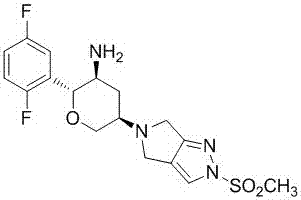
Synthesizing method of omarigliptin intermediate
A synthesis method and intermediate technology are applied in the synthesis field of alogliptin intermediates, which can solve the problems of low product yield, high cost, complexity and the like, and achieve a simple and easy synthesis method, good product quality and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of compound (4)
[0024] Add 2.18 kg (12 mol) of compound (3) and 710 g (10 mol) of compound (2) in 20L of anhydrous tetrahydrofuran to a 50L reactor at room temperature, and slowly add 27 g (0.05 mol) of catalyst. After stirring for half an hour, 1.94 kg (15 mol) of diisopropylethylamine was slowly added. After the reaction was monitored by TLC, 20L of water was slowly added, stirred and crystallized, filtered, the filter cake was washed with 10L of water, and dried to obtain compound (4) The crude product was recrystallized twice from isopropyl acetate to obtain 2.07 kg (8.87 mol) of the refined product of compound (4), with a yield of 88.7%. HPLC detection purity: 98.1%.
[0025] 1 H NMR (400 MHz, DMSO- d 6 ) δ 7.85 (s, 1H), 4.66 (s, 2H), 2.78-2.71 (m, 2H), 2.59-2.52 (m, 4H), 2.07-2.00 (m, 1H), 1.91-1.82 (m, 1H ).
[0026] ESI+ [M+H] + =234.
[0027] Preparation of compound (6)
[0028] Under nitrogen protection, 1.93 kg (8.3 mol) of compound (4)...
Embodiment 2
[0036] Preparation of compound (4)
[0037] According to the method of Example 1, the reaction solvent was replaced by anhydrous toluene; the reaction temperature was 110°C, and the molar ratio of compound (2) and compound (3) was 1:1; the base used in the reaction was replaced by NaHMDS. The molar ratio of compound (2) to catalyst was 200:1, and finally the refined product of compound (4) was obtained with a yield of 87.4%. HPLC detection purity: 97.8%.
[0038] Preparation of compound (6)
[0039] According to the method in Example 1, the solvent is replaced by anhydrous 2-methyltetrahydrofuran, the reaction temperature is 20-80°C, the preferred temperature is 65-75°C, and the molar ratio of compound (4) to catalyst is 100:1, and finally The refined product of compound (6) has a yield of 92.6%. Purity by HPLC: 97.2%.
[0040] Preparation of compound (1)
[0041] According to the method in Example 1, the reaction solvent was replaced by anhydrous acetone; the reaction te...
Embodiment 3
[0043] Preparation of compound (4)
[0044] According to the method of Example 1, the reaction solvent was replaced by anhydrous dichloromethane; the reaction temperature was 40°C, the molar ratio of compound (2) to compound (3) was 1:2.0; the molar ratio of compound (2) to catalyst was 200 : 1~20, the refined product of compound (4) was finally obtained, and the yield was 89.3%. HPLC detection purity: 98.8%.
[0045] Preparation of compound (6)
[0046] According to the method of Example 1, the solvent was replaced by anhydrous ether, the reaction temperature was 0°C, the molar ratio of compound (4) to catalyst was 100:10, and finally the refined product of compound (6) was obtained with a yield of 93.6%. HPLC detection purity: 97.9%.
[0047] Preparation of compound (1)
[0048] According to the method of Example 1, the reaction solvent was replaced by methanol; the reaction temperature was 0°C, and finally the refined product of compound (1) was obtained with a yield of 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com