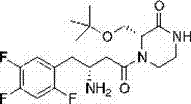
Application of asymmetric hydrogenation reaction in evogliptin synthesis
A hydrogenation reaction, asymmetric technology, applied in the direction of organic chemistry, can solve the problems of low product yield, high cost, poor quality, etc., and achieve the effect of simple synthesis method, good product quality and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0027] Preparation of compound (4)
[0028] Add 20 L of reaction solvent anhydrous dichloromethane and 2.5 kg (11 mol) of compound (2) into a 50L reaction kettle at 0°C, then slowly add 1.5 kg (11 mol) of oxalyl chloride (3 hours), and keep the temperature At about 0°C, TLC followed the reaction after the addition, and the reaction ended after 3 hours.
[0029] Add 10 L of reaction solvent anhydrous dichloromethane and 1.86kg (10 mol) of compound (3) to another 50L reaction kettle at 0°C, then add 1.11 kg (11 mol) of triethylamine, and then slowly add the above Acyl chloride, keep the temperature at about 0°C, follow the reaction by TLC after the addition is complete, add the reaction solution to 20L 5% sodium bicarbonate aqueous solution after 3 hours, separate the liquid, and concentrate the organic phase under reduced pressure to obtain compound (4) 3.76 kg (9.39 mol), the yield was 93.9%. HPLC detection purity: 97.8%.
[0030] 1 H NMR (400 MHz, DMSO- d 6 ) δ 7.47 – 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com