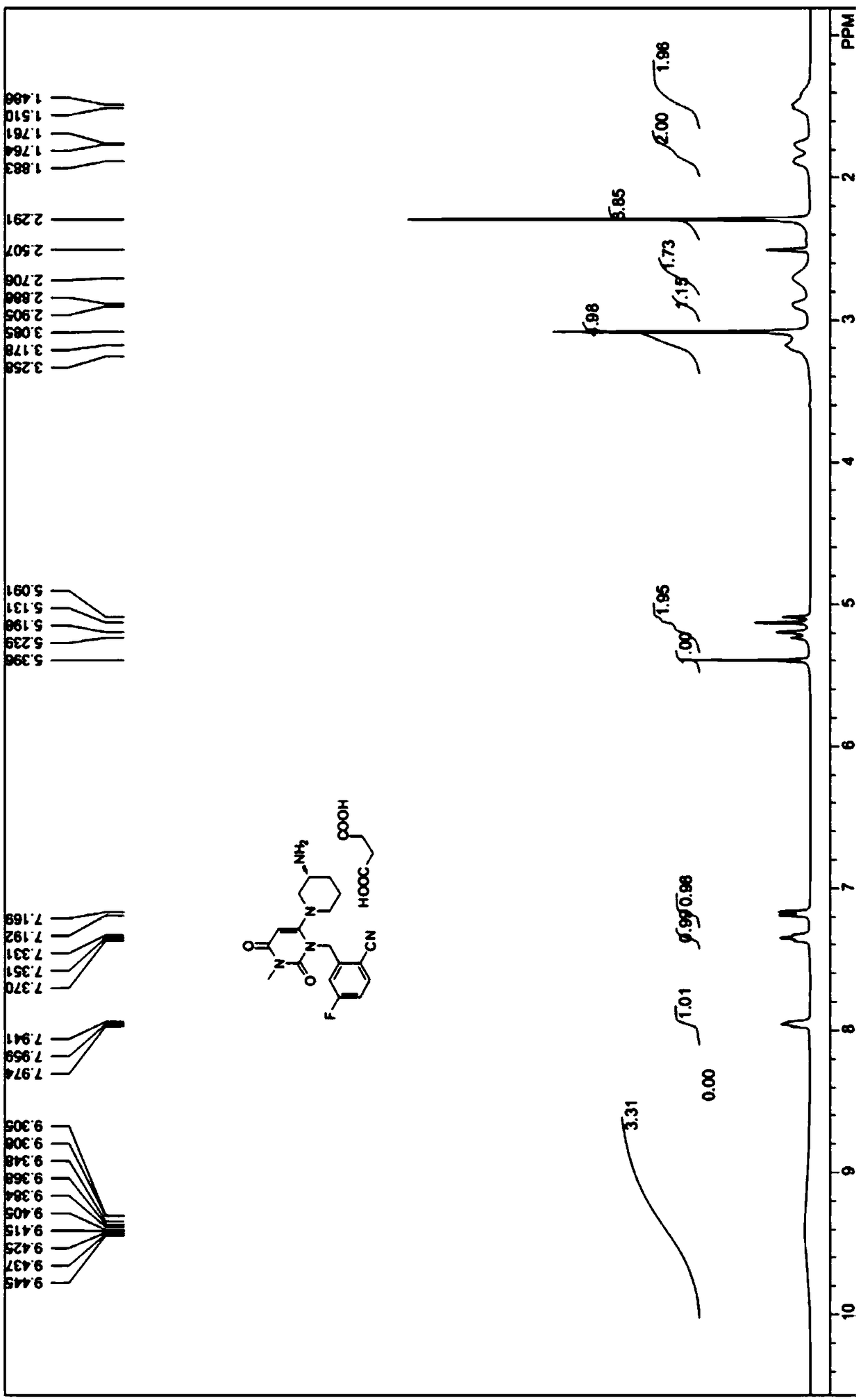
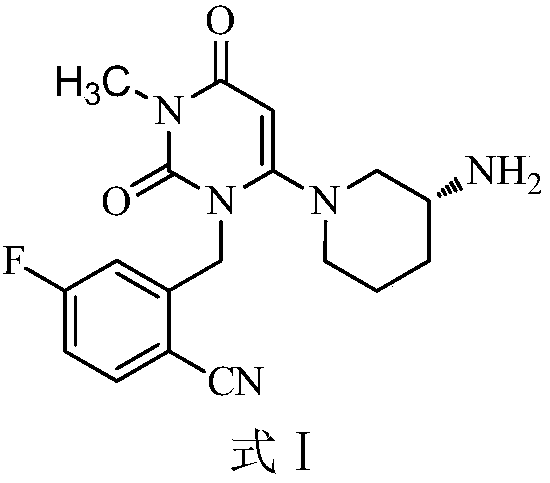
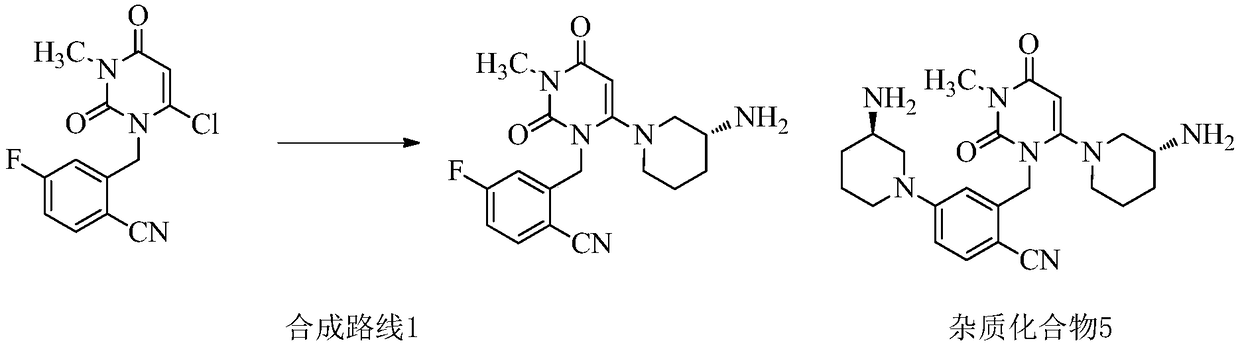
Preparation method of trelagliptin and salt of trelagliptin
A technology of methylation and dioxo generation, which is applied in the field of preparation of trexagliptin and its salts, can solve the problems of cumbersome separation and purification steps of crude products, unsuitability for industrial production, low yield of trexagliptin, etc., and achieve reduction of side effects The effect of reaction and formation of impurity compounds, low cost, and simple separation and purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] 6-Chloro-3-methyluracil (1.5g), potassium carbonate (3.88g), 2-cyano-5-fluorobenzyl bromide (2.6g) were dissolved in 20mL of DMSO, and the above mixture was heated to 50-60°C for 5h , cooled to 10°C, added 20 mL of water to the reaction solution, a light yellow solid precipitated, filtered, washed the solid with isopropanol, and dried in vacuo to obtain 1.6 g of the product (compound 3);
[0064] Under nitrogen protection, compound 3 (1g, 3.41mmol) was dissolved in 10mL DMSO, stirred until clear, and (Pd(OAc) 2 (7.7mg, 0.034mmol), BINAP (32mg, 0.051mmol), K 3 PO 4 (2.17g, 10.23mmol), the above mixture was heated to 80°C, 3-Boc-aminopiperidine (compound 7, 0.82g, 4.1mmol) was dissolved in 1mL DMSO and added to the above reaction mixture, stirred at 80°C for 5h, TLC Detect the disappearance of compound 3; cool down to room temperature, filter the reaction solution, pour the filtrate into water, extract with dichloromethane, dry the organic phase, and evaporate to drynes...
Embodiment 2
[0074] 6-Chloro-3-methyluracil (30g), potassium carbonate (77.6g), 2-cyano-5-fluorobenzyl bromide (52g) were dissolved in 200mL of DMSO, and the above mixture was heated to 50-60°C for 5h, then cooled After reaching 10°C, 200 mL of water was added to the reaction solution, and a pale yellow solid precipitated out. After filtration, the solid was washed with isopropanol and dried in vacuo to obtain 33 g of the product (compound 3);
[0075] Under nitrogen protection, compound 3 (10g, 34.1mmol) was dissolved in 50mL DMSO, stirred until clear, and Pd(OAc) (77mg, 0.34mmol), BINAP (320mg, 0.51mmol), K 3 PO 4 (21.7g, 102.3mmo), the above mixture was heated to 80°C, 3-Boc-aminopiperidine (compound 7, 8.2g, 40.9mmol) was dissolved in DMSO (10ml) and added to the above reaction mixture, stirred at 80°C for 5h , TLC detects that compound 3 disappears; cool down to room temperature, filter the reaction solution, pour the filtrate into water, extract with dichloromethane, dry the organi...
Embodiment 3
[0079] 6-Chloro-3-methyluracil (3g, 18.8mmol), potassium carbonate (12.9g, 94mmol), 2-cyano-5-fluorobenzyl bromide (6g, 28.1mmol) were dissolved in 60mL of DMSO, and the above mixture was heated React at 50-60°C for 5h, cool down to 10°C, add 60mL of water to the reaction solution, and a pale yellow solid precipitates out. After filtration, the solid was washed with isopropanol and dried in vacuo to obtain 2.8 g of the product (compound 3);
[0080] Under nitrogen protection, compound 3 (2g, 6.8mmol) was dissolved in 20mL DMSO, stirred until clear, and Pd(OAc) (153mg, 0.68mmol), BINAP (622mg, 1mmol), K 3 PO 4 (4.3g, 20.4mmol), the above mixture was warmed to 80°C, (R)-3-Boc-aminopiperidine (compound 7, 2g, 10.2mmol) was dissolved in DMSO (50ml) and added to the above reaction mixture, 80 Stir at ℃ for 5 h, TLC detects that compound 3 disappears; cool down to room temperature, filter the reaction solution, pour the filtrate into water, extract with dichloromethane, dry the or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com