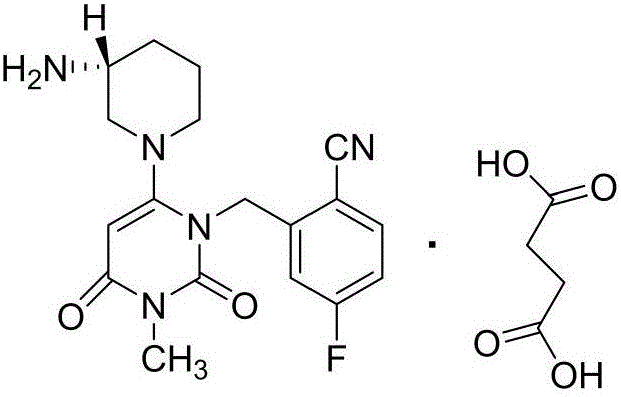
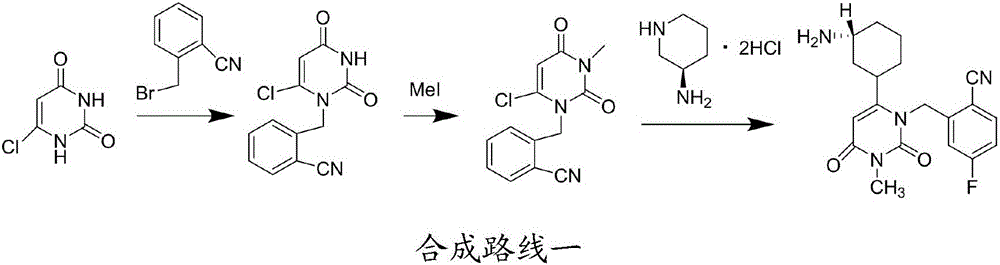
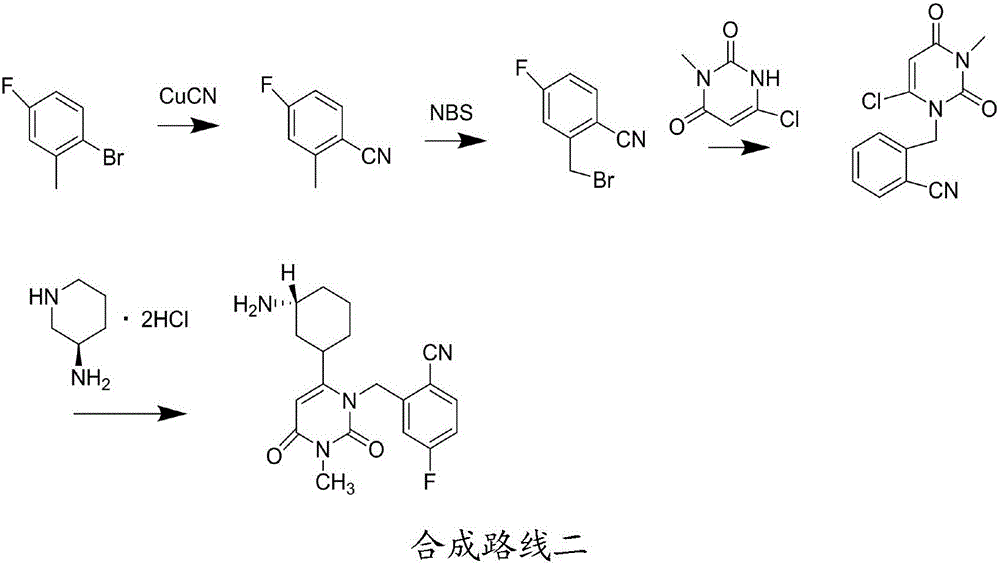
Preparation method for degraded trelagliptin impurity
A technology of impurities and methyl groups, which is applied in the preparation of trexagliptin to degrade impurities, and can solve problems such as the impact on drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 2-[[6-[(3R)-3-amino-1-piperidinyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl ]Methyl]-5-fluorobenzonitrile (20.0g, 39.8mmol) was added to 100ml of dichloromethane, 30% hydrogen peroxide (22.5g, 199mmol) was added, then concentrated sulfuric acid (0.8g, 8.0mmol) was added and phase Transfer catalyst tetrabutylammonium bromide (1.29g, 4.0mmol), react at 30°C for 20h. After the reaction was completed, the pH of the reaction solution was adjusted to about 8 with saturated sodium carbonate solution, and the layers were separated. The aqueous phase was extracted with dichloromethane (30ml×3), and the organic phases were combined and concentrated under reduced pressure. Silica gel column chromatography (developing solvent: dichloromethane: isopropanol = 4:1) gave 15.2 g of a white solid, namely 2-[[6-[(3R)-3-amino-1-piperidinyl]- 3,4-Dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl]methyl]-5-fluorobenzamide, yield 73.3%, purity 98.9%.
[0043] 1 H-NMR(DMSO,400M)δ:8.41(s,...
Embodiment 2
[0046] 2-[[6-[(3R)-3-amino-1-piperidinyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl ]Methyl]-5-fluorobenzonitrile (20.0g, 39.8mmol) was added in 100ml of dichloromethane, 30% hydrogen peroxide (22.5g, 199mmol) was added, then formic acid (0.9g, 19.9mmol) was added and phase transfer Catalyst Tetrabutylammonium bromide (1.29g, 4.0mmol), react at 30°C for 30h. After the reaction was completed, the pH of the reaction solution was adjusted to about 8 with saturated sodium carbonate solution, and the layers were separated. The aqueous phase was extracted with dichloromethane (30ml×3), and the organic phases were combined and concentrated under reduced pressure. Silica gel column chromatography (developing solvent: dichloromethane: isopropanol = 4:1) gave 14.8 g of a white solid, namely 2-[[6-[(3R)-3-amino-1-piperidinyl]- 3,4-Dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl]methyl]-5-fluorobenzamide, yield 71.3%, purity 98.2%.
Embodiment 3
[0048] 2-[[6-[(3R)-3-amino-1-piperidinyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl ]Methyl]-5-fluorobenzonitrile (20.0g, 39.8mmol) was added to 100ml of ethanol, 30% hydrogen peroxide (22.5g, 199mmol) was added, concentrated sulfuric acid (0.8g, 8.0mmol) was added, and the reaction was carried out at 30°C 20h. After the reaction, adjust the pH of the reaction solution to about 8 with saturated sodium carbonate solution, concentrate under reduced pressure, add 50ml of water and 100ml of dichloromethane and stir for 10min, separate layers, extract the aqueous phase with dichloromethane (30ml×3), and combine the organic phases , concentrated under reduced pressure. Silica gel column chromatography (developing solvent: dichloromethane: isopropanol = 4:1) gave 15.6 g of a white solid, namely 2-[[6-[(3R)-3-amino-1-piperidinyl]- 3,4-Dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl]methyl]-5-fluorobenzamide, yield 75.2%, purity 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com