Oral tablet containing trelagliptin succinate and preparation method thereof
A technology of trilagliptin succinate and oral tablet is applied in the field of oral tablet containing trilagliptin succinate and its preparation field, which can solve the problem that the crude drug of trilagliptin succinate is easy to aggregate and is not suitable for commercialization Production, granulation and other problems are not good, to achieve good in vitro dissolution behavior, to solve the effect of poor fluidity and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
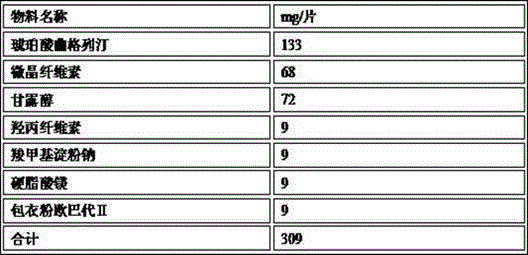
[0047]
[0048] Note: Trexagliptin succinate particle size d0.9=79μm
[0049] The preparation method is as follows: mix troxagliptin succinate, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and croscarmellose sodium and sieve to obtain mixed powder 1; mix powder 1, part The magnesium stearate is mixed for later use to obtain mixed powder 2; the mixed powder 2 is dry-granulated by a dry granulator to obtain dry granules of troxagliptin succinate; the dry granules of troxagliptin succinate and remaining stearin Magnesium acid mixed evenly, compressed into tablets.
[0050] Comparative Example 1, Comparative Example 2, and Example 1 were placed naked under high-temperature and high-humidity conditions, and the related substances on day 0 and day 10 were investigated. The results are shown in Table 1.
[0051] Table 1: Related Substance Results
[0052]
Embodiment 2
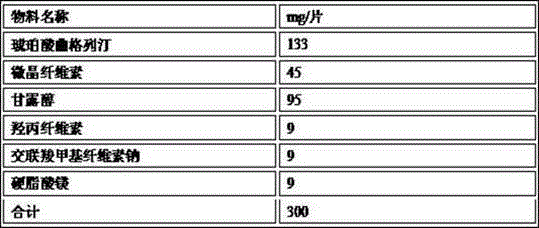
[0055]
[0056] Note: Trexagliptin succinate particle size d0.9=79μm
[0057] The preparation method is as follows: mix troxagliptin succinate, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and croscarmellose sodium and sieve to obtain mixed powder 1; mix powder 1, part The magnesium stearate is mixed for later use to obtain mixed powder 2; the mixed powder 2 is dry-granulated by a dry granulator to obtain dry granules of troxagliptin succinate; the dry granules of troxagliptin succinate and remaining stearin Magnesium acid mixed evenly, compressed into tablets.
Embodiment 3
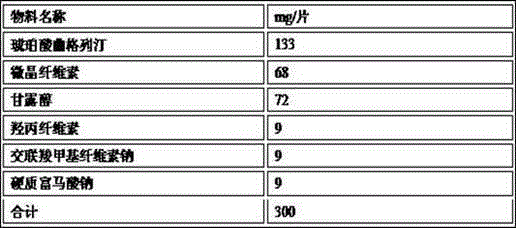
[0059]
[0060] The preparation method is as follows: mix troxagliptin succinate, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and croscarmellose sodium and sieve to obtain mixed powder 1; mix powder 1, part The hard sodium fumarate is mixed and set aside to obtain mixed powder 2; the mixed powder 2 is dry-granulated by a dry granulator to obtain troxagliptin succinate dry granules; the troxagliptin succinate dry granules and the remaining Hard sodium fumarate is mixed evenly and pressed into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com