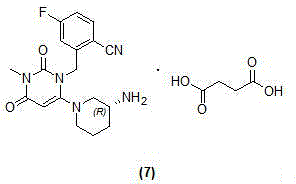
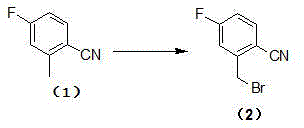Industrialization-suitable preparation method of high-purity trelagliptin succinate
A technology of troxagliptin succinate and succinic acid, which is applied in the field of preparation of troxagliptin succinate, can solve the problems of difficult to obtain high-purity products and easy to produce more impurities, and achieve easy operation and low impurity Less, the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of 2-bromomethyl-4-fluorobenzonitrile:
[0042] A mixture of 4-fluoro-2-methylbenzonitrile (2g, 14.8mmol), N-bromosuccinimide (NBS) (2.64g, 15mmol) and AIBN (0.10g) in dichloromethane was refluxed for 1h. The reaction was cooled to room temperature. Wash with sodium bisulfite solution, sodium carbonate solution, and saturated sodium chloride solution. The organic solvent was concentrated to obtain 2.5 g of oily 2-bromomethyl-4-fluorobenzonitrile (yield 87%).
[0043] Amino-1-piperidine)-3-methyl-2,4 ( 1H, 3H )-Synthesis of dihydropyrimidinedione:
[0044] Combine 3-methyl-6-chlorouracil (0.6g, 3.8mmol), (R)-3-(trifluoroacetyl-)amino-piperidine (0.8g, 4.0mmol) and DIPEA (1.4ml, 8mmol) ) The mixture in absolute ethanol (10ml) is stirred at 80°C until the reaction is complete. Add water, the solid will separate out and filter. 1.11 g of 6-[3-(trifluoroacetyl-)amino-1-piperidine]-3-methyl-2,4( 1H, 3H )-Dihydropyrimidinedione (92.4% yield).
[0045] (R)-2-[(6-(3-A...
Embodiment 2
[0050] Synthesis of 2-bromomethyl-4-fluorobenzonitrile:
[0051] A mixture of 4-fluoro-2-methylbenzonitrile (2g, 14.8mmol), bromine (2.64g, 15mmol) and benzoyl peroxide (BPO) (0.10g) in chloroform was refluxed for 6h. The reaction was cooled to room temperature. Wash with sodium bisulfite solution, sodium carbonate solution, and saturated sodium chloride solution. The organic solvent was concentrated to obtain 2.5 g of oily 2-bromomethyl-4-fluorobenzonitrile (yield 86.3%).
[0052] Amino-1-piperidine)-3-methyl-2,4 ( 1H, 3H )-Synthesis of dihydropyrimidinedione:
[0053] Combine 3-methyl-6-chlorouracil (0.6g, 3.8mmol), (R)-3-(phthaloyl-)amino-piperidine (0.8g, 4.0mmol) and triethylamine (1.4 ml, 8mmol) in absolute ethanol (10ml) was stirred at 60°C until the reaction was complete. Add water, the solid will separate out and filter. 1.11 g of 6-[3-(phthaloyl-)amino-1-piperidine]-3-methyl-2,4( 1H, 3H )-Dihydropyrimidinedione (92.1% yield).
[0054] (R)-2-[(6-(3-Amino-piperidin-1-...
Embodiment 3
[0059] Synthesis of 2-bromomethyl-4-fluorobenzonitrile:
[0060] A mixture of 4-fluoro-2-methylbenzonitrile (2g, 14.8mmol), N-bromosuccinimide (NBS) (2.64g, 15mmol) and AIBN (0.10g) in chloroform was refluxed for 2h. The reaction was cooled to room temperature. Wash with sodium bisulfite solution, sodium carbonate solution, and saturated sodium chloride solution. The organic solvent was concentrated to obtain 2.88 g of oily 2-bromomethyl-4-fluorobenzonitrile (yield 91%).
[0061] Amino-1-piperidine)-3-methyl-2,4 ( 1H, 3H )-Synthesis of dihydropyrimidinedione:
[0062] Combine 3-methyl-6-chlorouracil (0.6g, 3.8mmol), (R)-3-(tert-butoxycarbonyl-)amino-piperidine (0.8g, 4.0mmol) and DIPEA (1.4ml, 8mmol) ) The mixture in absolute ethanol (10ml) is stirred at 70°C until the reaction is complete. Add water, the solid will separate out and filter. 1.15 g of 6-[3-(tert-butoxycarbonyl-)amino-1-piperidine]-3-methyl-2,4( 1H, 3H )-Dihydropyrimidinedione (93.5% yield).
[0063] (R)-2-[(6-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com