Preparation method of trelagliptin succinate
A technology of trelagliptin succinate and succinic acid, applied in the field of preparation of trelagliptin succinate, can solve problems such as unfavorable intermediate MI purification, failure to effectively control isomer impurities, unfavorable amplification of production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
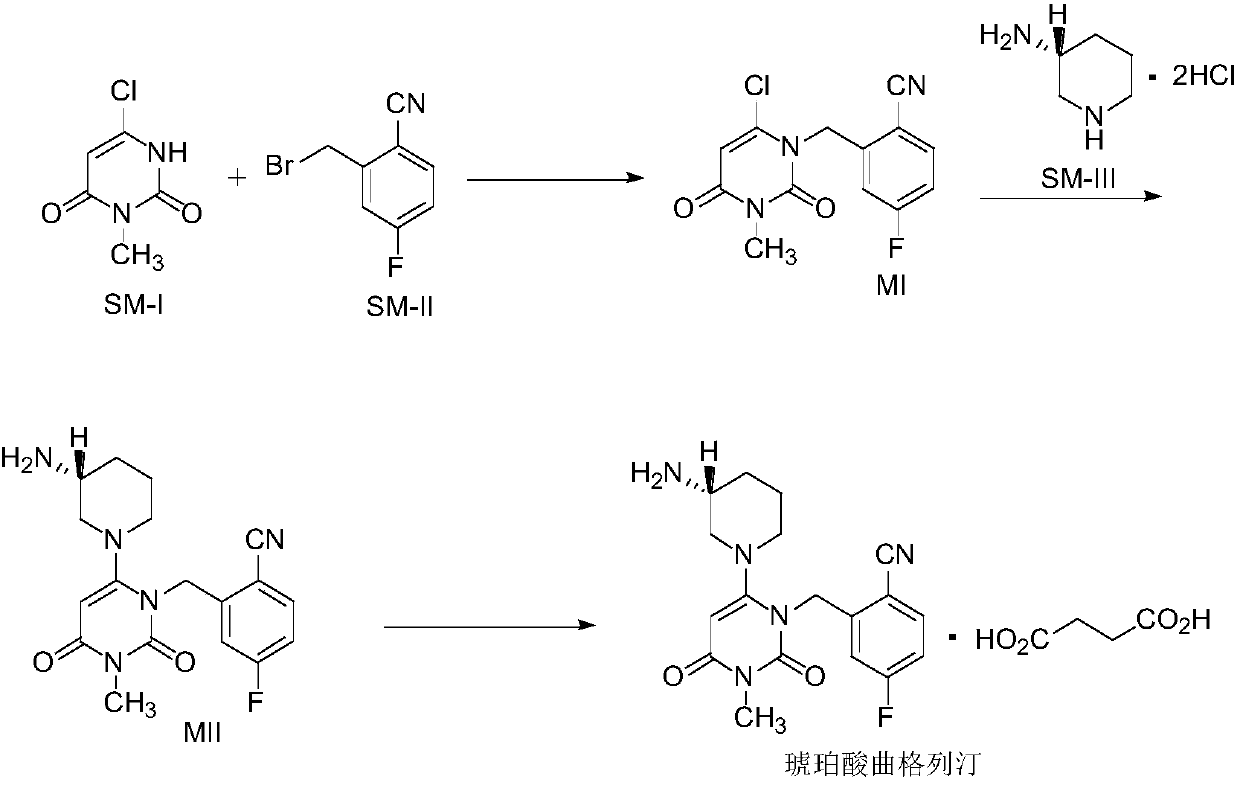
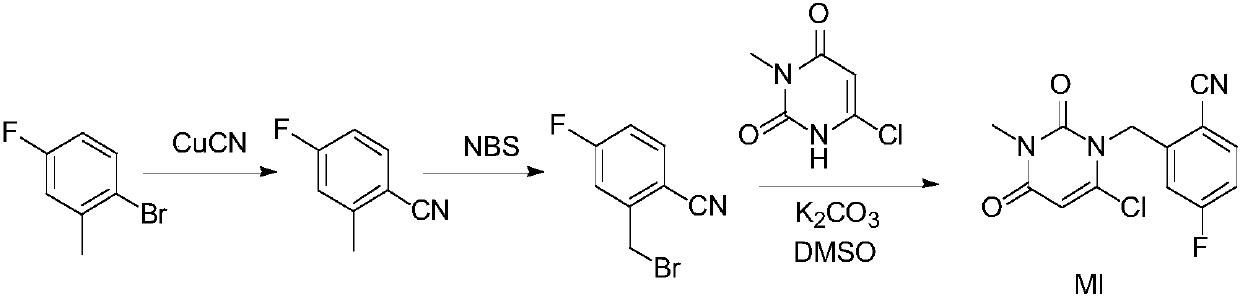
[0045] Embodiment 1: the preparation of intermediate MI
[0046]
[0047]Add toluene into the 200L reactor, start stirring, and add 16.0kg of 6-chloro-3-methyluracil, 23.5kg of 2-cyano-5-fluorobenzyl bromide and 27.7kg of tri-n-butylamine in sequence. Under stirring, raise the temperature of the mixture to 80±5°C, keep the reaction at this temperature for about 6 hours, monitor the reaction by TLC (petroleum ether: ethyl acetate = 3:2), when 6-chloro-3-methyluracil is basically consumed Completely both as the end point of the reaction.
[0048] After the reaction is complete, add 48.0L of purified water to the reaction kettle, cool down to 20±5°C, stir for 0.5h to 1h, centrifuge, wash the filter cake twice with purified water (24.0L×2), centrifuge, and collect the filter cake , transfer the filter cake to a 200L reaction kettle, add 64.0L of isopropanol, beat at room temperature for 1 to 2 hours, centrifuge, and stir the filter cake twice with isopropanol (16.0L×2), collec...
Embodiment 2
[0053] Embodiment 2: the preparation of intermediate MII
[0054]
[0055] Add 260.0L of isopropanol and 36.4L of purified water into the 800L reactor, start stirring, and then add 26.0kg of intermediate MI, 22.9kg of (R)-3-aminopiperidine dihydrochloride and 32.8kg of anhydrous sodium carbonate . The mixture was heated to 70±5°C under stirring, and kept at this temperature for about 18 hours. The reaction was monitored by TLC (dichloromethane:methanol=20:1), and the end point of the reaction was when the intermediate MI was basically completely consumed.
[0056] After the reaction is complete, add 260.0 L of ethyl acetate into the reaction kettle, cool down to 20±5°C, stir for 0.5-1 h, centrifuge, collect the filtrate, and filter cake for later use. Add the filtrate into an 800L reaction kettle, slowly add an appropriate amount of concentrated hydrochloric acid under stirring, adjust the pH to 3-5, and precipitate a solid. Stir for 0.5h and centrifuge to collect the sol...
Embodiment 3
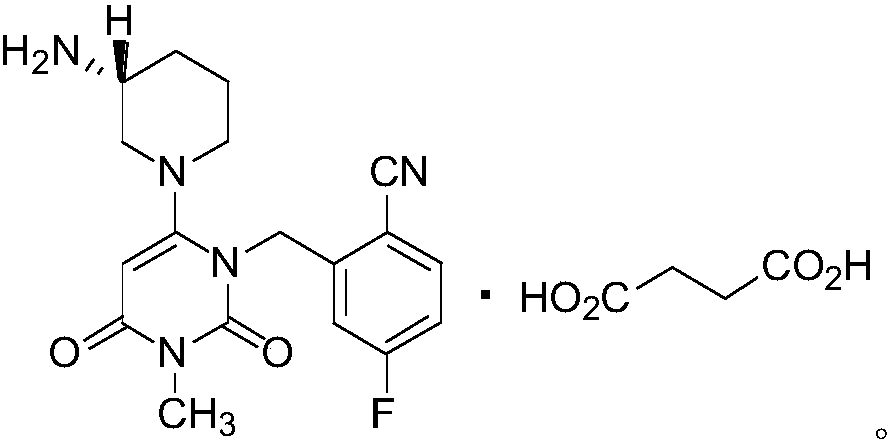
[0063] Embodiment 3: the preparation of trexagliptin succinate
[0064]
[0065] Add 102.5L of absolute ethanol to the 200L reactor, and add 20.5kg of intermediate MII under stirring. Raise the temperature to 80±5°C to dissolve it, inject it into a 500L reactor in the clean area while it is hot, and keep stirring at 80±5°C; add 102.5L of absolute ethanol to another 200L reactor, and add 7.18kg of succinic acid while stirring. Raise the temperature to 80±5°C to dissolve it, and slowly inject it into a 500L reactor in the clean area while it is still hot. Solids will precipitate during the addition process. After the addition is complete, keep warm at 80±5°C for 6 hours. Then lower the temperature to 20±5°C, stir for 1-2h, centrifuge, stir and wash the filter cake with 41.0L of absolute ethanol, centrifuge, collect the filter cake, and vacuum-dry at 50±5°C to obtain the finished product of Trexagliptin succinate ( 26.20kg, yield: 96%, purity: 99.9%).
[0066] 1 H NMR (500M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com