Trelagliptin succinate tablets
A technology of troxagliptin succinate and tablet, applied in the field of medicine, can solve the problems of slow disintegration and difficult absorption of tablets, and achieve the effects of good stability, easy operation and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
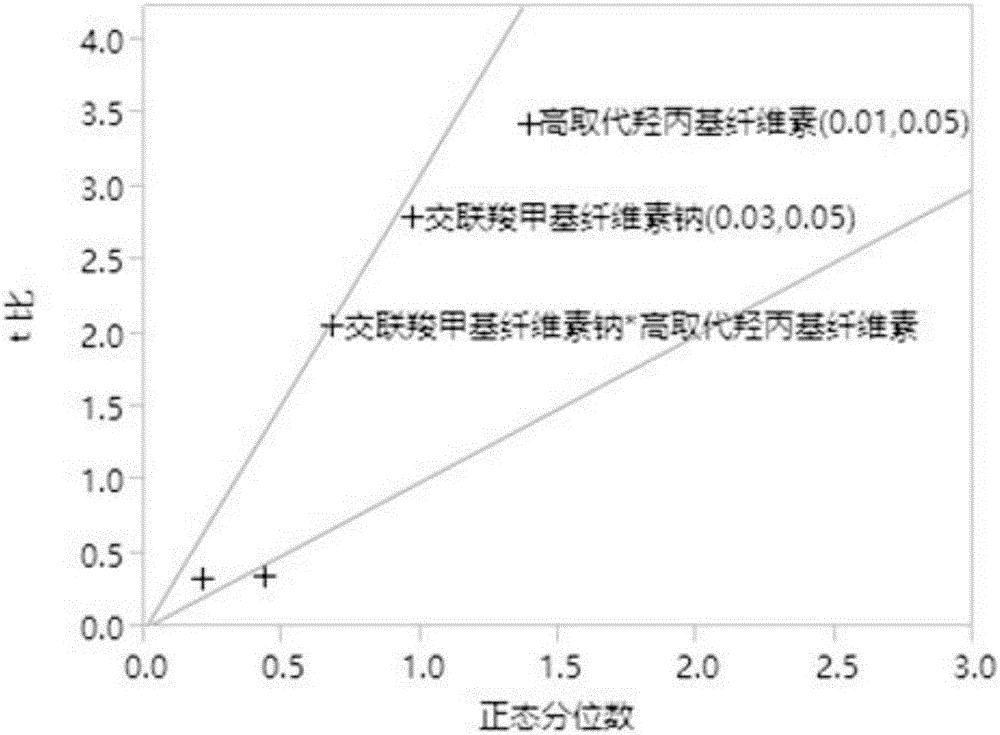
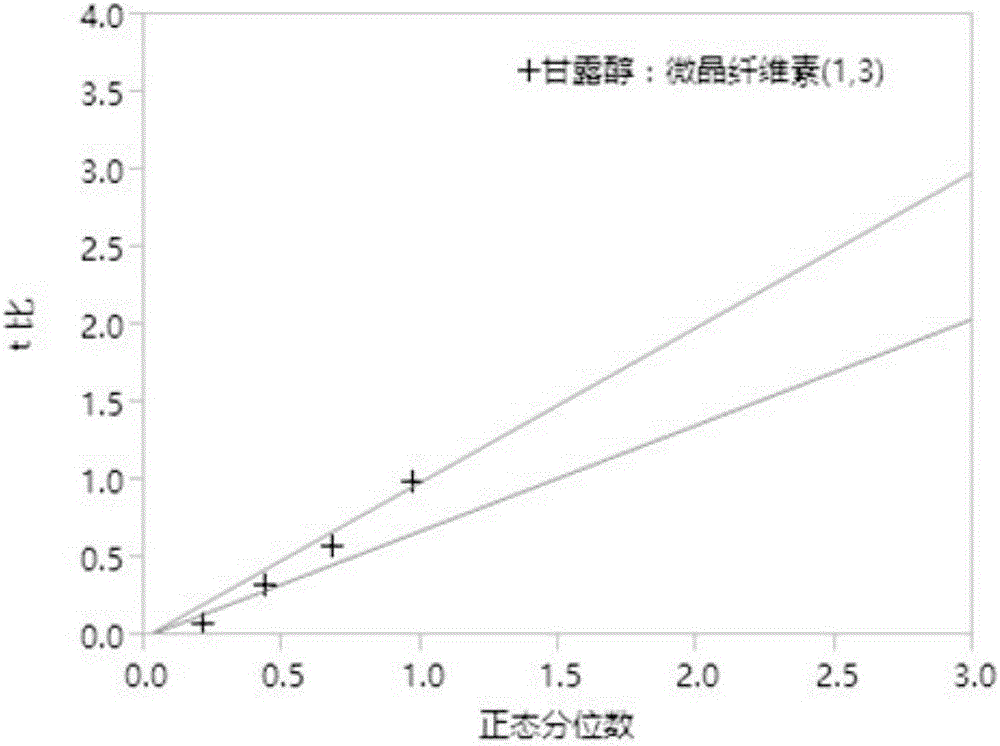
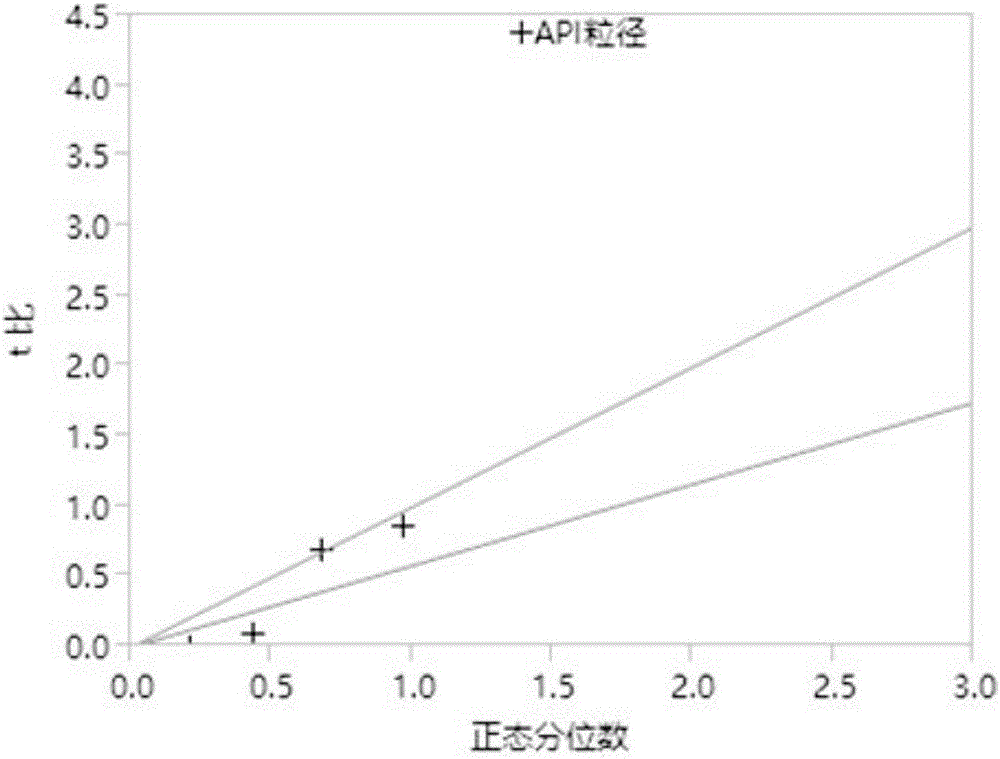
[0032] Example 1 Desagliptin Succinate Tablet Prescription Screening
[0033] This experiment is an optimization design of the prescription of trexagliptin succinate tablet based on the Screen method, as follows:
[0034] 1. Risk assessment of Trexagliptin raw material
[0035] The properties of trexagliptin raw materials may have an impact risk assessment on the critical quality attributes (CQAs) of the preparation. Rank the relative risk assessed for each attribute as high, medium, or low. High-risk attributes require in-depth research in the preparation development process, thereby reducing the risk assessment level; medium risk assessment levels need to determine the acceptance of the current risk level or conduct appropriate research to reduce the risk according to the needs of the research; The CQAs of the formulation had basically no effect, and no further study was required. The following table 1 is based on the physical and chemical properties, biopharmaceutical pr...
Embodiment 2
[0068] The preparation of embodiment 2 trexagliptin succinate tablet
[0069] prescription
[0070]
[0071] Preparation method: mix troxagliptin succinate, croscarmellose sodium, hydroxypropyl cellulose, mannitol and microcrystalline cellulose evenly, pass through an 80-mesh sieve for later use, and carry out dry granulation through a dry granulator , to obtain dry granules; mix the dry granules with sodium stearyl fumarate evenly, and press into tablets.
Embodiment 3
[0072] The stability of embodiment 3 trexagliptin succinate tablet
[0073] The Trexagliptin succinate tablets prepared in Example 2 were placed under high temperature and high humidity conditions, and the related substances on day 0 and day 10 were investigated. The results are shown in Table 8.
[0074] Table 8 Stability of Trexagliptin Succinate Tablets
[0075]
[0076]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com