Preparation method of composite trelagliptin succinate tablets
A technology of troxagliptin succinate and compound succinic acid is applied in the field of medicine to achieve the effects of treating hyperglycemia, less toxic and side effects, and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
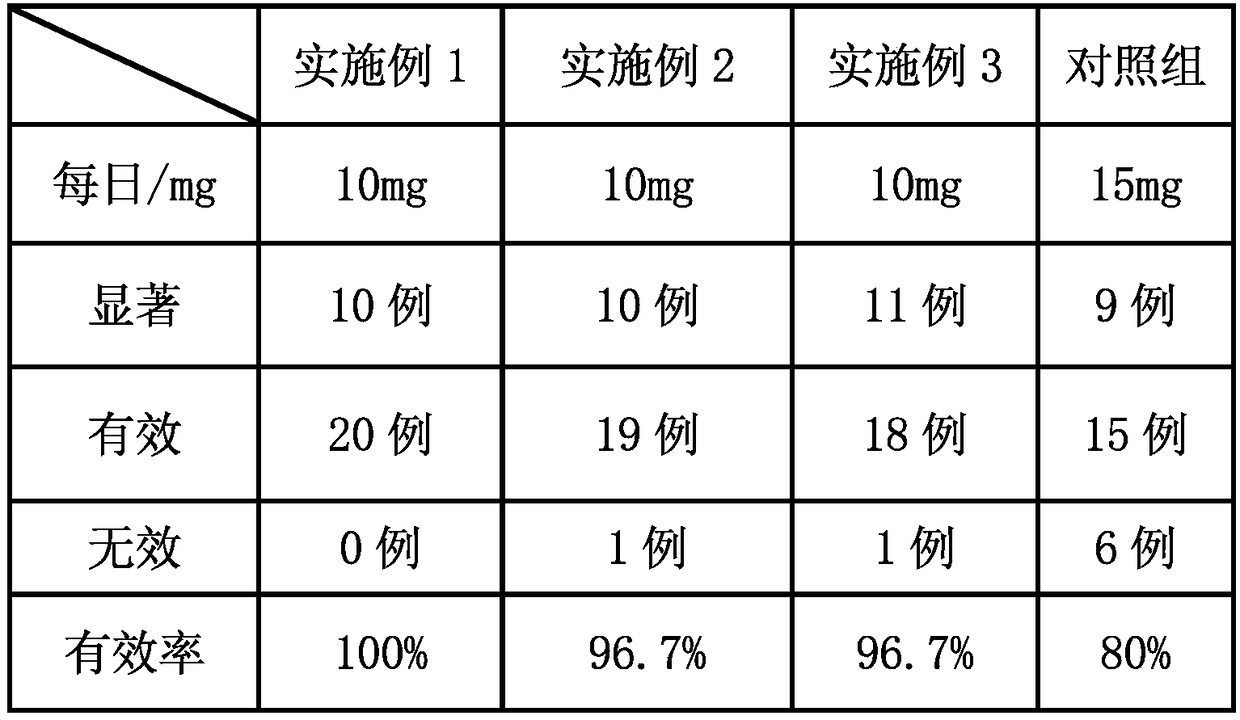
Embodiment 1
[0043] A kind of preparation method of composite troxagliptin succinate tablet, the preparation of troxagliptin succinate raw material, comprises the following steps:
[0044] Step 1: Intermediate 2-[(6-chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]-4-fluorobenzyl Nitrile preparation:
[0045] Add 10.8kg of ethyl acetate, 2.4kg of 2-bromomethyl-4-fluorobenzonitrile, 1.74kg of 6-chloro-3-methyluracil, and 2.27kg of triethylamine to the 20L reactor in sequence; start stirring and heating The device is heated to an internal temperature of 60-70°C; the temperature range in the reaction kettle is controlled to 60-70°C, and the reaction time is 1.5-2.5 hours.
[0046] Reaction end point control method: use thin-layer chromatography, choose petroleum ether: ethyl acetate = 3: 1 (PE: EA = 3: 1) as the developer ratio, develop color under ultraviolet light, until the raw material spots disappear, it is regarded as The reaction is complete;
[0047] After the reactio...
Embodiment 2
[0079] A kind of preparation method of composite troxagliptin succinate tablet, the preparation of troxagliptin succinate raw material, comprises the following steps:
[0080] Step 1: Intermediate 2-[(6-chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]-4-fluorobenzyl Nitrile preparation:
[0081] Add 9.6kg of ethyl acetate, 1.9kg of 2-bromomethyl-4-fluorobenzonitrile, 1.24kg of 6-chloro-3-methyluracil, and 1.87kg of triethylamine to the 20L reactor in sequence; start stirring and heating The device is heated to an internal temperature of 60-70°C; the temperature range in the reaction kettle is controlled to 60-70°C, and the reaction time is 1.5-2.5 hours.
[0082] Reaction end point control method: use thin-layer chromatography, choose petroleum ether: ethyl acetate = 3: 1 (PE: EA = 3: 1) as the developer ratio, develop color under ultraviolet light, until the raw material spots disappear, it is regarded as The reaction is complete;
[0083]After the reaction ...
Embodiment 3
[0115] A kind of preparation method of compound troxagliptin succinate tablet, the preparation of troxagliptin succinate raw material, comprises the following steps:
[0116] Step 1: Intermediate 2-[(6-chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]-4-fluorobenzyl Nitrile preparation:
[0117] Add 11.3kg of ethyl acetate, 2.9kg of 2-bromomethyl-4-fluorobenzonitrile, 2.24kg of 6-chloro-3-methyluracil, and 2.57kg of triethylamine to the 20L reactor in sequence; start stirring and heating The device is heated to an internal temperature of 60-70°C; the temperature range in the reaction kettle is controlled to 60-70°C, and the reaction time is 1.5-2.5 hours.
[0118] Reaction end point control method: use thin-layer chromatography, choose petroleum ether: ethyl acetate = 3: 1 (PE: EA = 3: 1) as the developer ratio, develop color under ultraviolet light, until the raw material spots disappear, it is regarded as The reaction is complete;
[0119] After the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com