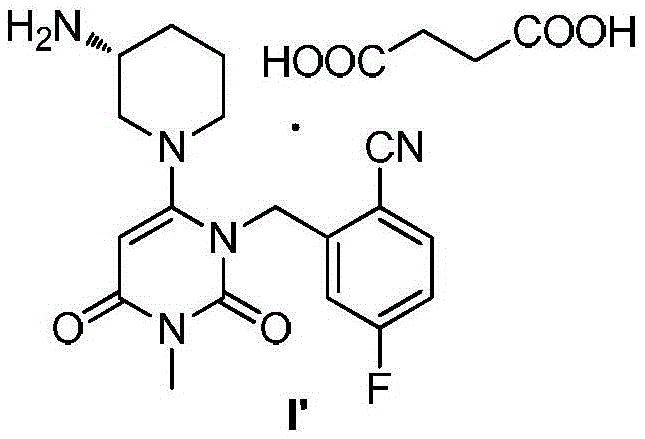
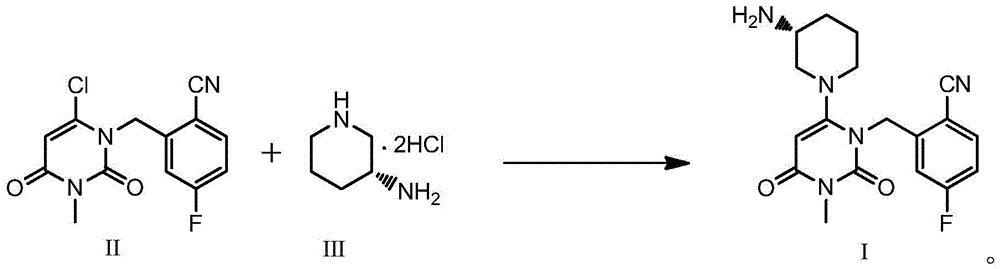Trelagliptin and preparation method of succinate thereof
A technology of troxagliptin succinate and succinic acid, which is applied in the field of preparation of troxagliptin and troxagliptin succinate, can solve the problems of low yield, high production cost, and complicated operation of the preparation method, and achieve The effect of reducing the content of impurities, low production cost, simple and safe process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
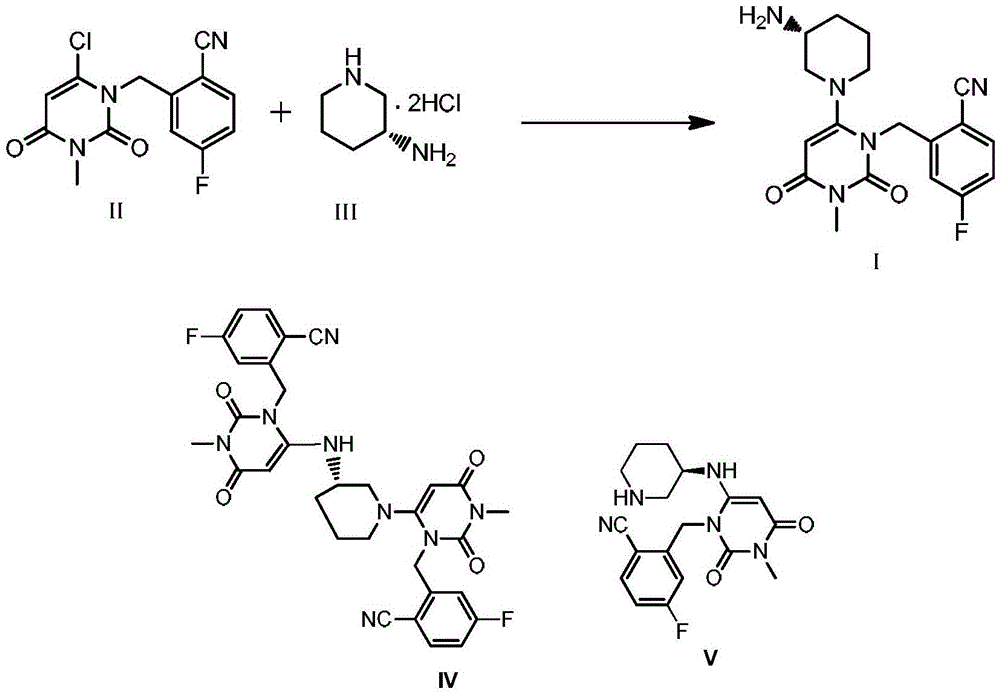
[0039] Embodiment 1: Preparation of Trexagliptin Intermediate II (with reference to the method reported by CN101573351A)
[0040]
[0041] 3.0 Kg of 4-fluoro-2-methylbenzonitrile was added to 23.7 Kg of acetonitrile, and then 4.0 Kg of N-bromosuccinimide and 0.15 Kg of azobisisobutyronitrile were added. Heated to 70°C for 8 hours. Concentrate under reduced pressure to remove acetonitrile, add 25Kg of dichloromethane and stir, filter and wash the filter cake with 12Kg of dichloromethane. Wash the filtrate three times with 15Kg of 7% sodium bisulfite solution; add 15Kg of N-methylpyrrolidone after concentrating the organic phase to remove dichloromethane, then add 3.03Kg of 6-chloro-3-methyluracil and 2.91Kg of potassium carbonate . Heated to 60°C for 5 hours. Cool to 35°C, add 45Kg of water, cool to 10°C, stir, and filter. Add 19Kg of n-heptane to the filter cake, stir, filter, and air-dry at 50-55°C to obtain trexagliptin intermediate II, 4.49Kg, yield: 68.9%, HPLC puri...
Embodiment 2
[0042] Embodiment 2: the preparation of trexagliptin succinate I '
[0043]
[0044] Trexagliptin intermediate II 4.49Kg is added isopropanol 35.5Kg, then adds trexagliptin intermediate III ((R)-3-aminopiperidine dihydrochloride) 2.91Kg, potassium phosphate 8.1Kg and four 11 g of n-propylammonium bisulfate was heated to 40°C to 50°C for 4 to 5 hours. After concentration under reduced pressure, 30 Kg of dichloromethane and 22.5 Kg of water were added to separate layers, and the organic phase was washed three times with water. The aqueous phase was extracted once with 12Kg of dichloromethane. Combine the organic phases, add 0.9 Kg of anhydrous sodium sulfate and let stand for 3 hours, filter, and concentrate under reduced pressure to obtain 4.48 Kg of trexagliptin crude product. Add 21.6Kg of methanol to the whole batch of troxagliptin crude product, raise the temperature to 60°C-65°C to clarify the solution, cool to 20°C-25°C and stir for 1 hour to 2 hours at a stirring ra...
Embodiment 3
[0046] Embodiment 3: the preparation of trexagliptin succinate I '
[0047] Add 10.0 g of trexagliptin intermediate II to 79 g of isopropanol, then add 9.4 g of trexagliptin intermediate III, 30.7 g of anhydrous sodium phosphate and 116 mg of tetra-n-butylammonium bisulfate, and heat to 30°C to 40°C. ℃ for 7 hours to 9 hours. After concentration under reduced pressure, 50 mL of dichloromethane and 50 mL of water were added to separate layers, and the organic phase was washed twice with water. The aqueous phase was extracted twice with 20 mL of dichloromethane. The organic phases were combined, 2 g of anhydrous magnesium sulfate was added and allowed to stand for 5 hours, filtered, and concentrated under reduced pressure to obtain 10.1 g of crude trexagliptin. Add 60mL of ethanol to the whole batch of crude trexagliptin, heat to 60°C-65°C and stir until clear, cool to 20°C-25°C and stir for 1 hour to 2 hours, filter, and use ethanol cooled to 10°C-15°C 5 mL was washed three ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com