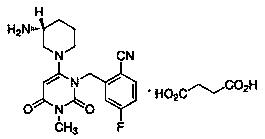
Succinic acid trelagliptin orally-disintegrating tablets and preparing method thereof
A technology of troxagliptin succinate and orally disintegrating tablets, which is applied in the directions of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. problem, to achieve the effect of easy swallowing, good promotion prospects, and short disintegration time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
1%
[0032] Preparation:
[0033] (1) Micronize trexagliptin succinate, grind the rest of the excipients separately and pass through a 100-mesh sieve;
[0034] (2) After sieving and mixing troxagliptin succinate, mannitol, lactose, carboxymethyl starch sodium of 5% prescription quantity and aspartame, add povidone ethanol aqueous solution with a prescription quantity of 20% , to prepare soft materials;
[0035] (3) Granulate through a 20-mesh sieve, dry in an oven at 50°C until the moisture content of the granules is between 1.5 and 3.5%, and pass through a 24-mesh sieve for granulation;
[0036] (4) Add micropowder silica gel, magnesium stearate, and the remaining amount of sodium carboxymethyl starch to the dry granules obtained in (3), and mix well;
[0037] (5) Determining the content of intermediates, determining the weight of the tablet and pressing it to obtain the tablet.
Embodiment 2
1%
[0040] Preparation:
[0041] (1) Micronize trexagliptin succinate, grind the rest of the excipients separately and pass through a 100-mesh sieve;
[0042] (2) After sieving and mixing troxagliptin succinate, mannitol, lactose, 5% croscarmellose sodium and aspartame in the prescribed amount, add 25% prescription amount of hydroxypropyl Cellulose-based ethanol aqueous solution to prepare soft materials;
[0043] (3) Granulate through a 20-mesh sieve, dry in an oven at 50°C until the moisture content of the granules is between 1.5 and 3.5%, and pass through a 24-mesh sieve for granulation;
[0044] (4) Add magnesium stearate and the remaining amount of croscarmellose sodium to the dry granules obtained in (3), and mix well;
[0045] (5) Determining the content of intermediates, determining the weight of the tablet and pressing it to obtain the tablet.
Embodiment 3
1%
[0048] Preparation:
[0049] (1) Micronize trexagliptin succinate, grind the rest of the excipients separately and pass through a 100-mesh sieve;
[0050] (2) After sieving and mixing troxagliptin succinate, mannitol, xylitol, crospovidone with a prescription volume of 3%, aspartame, and lemon essence, add 20% prescription volume Hydroxypropyl cellulose ethanol aqueous solution to prepare soft materials;
[0051] (3) Granulate through a 20-mesh sieve, dry in an oven at 50°C until the moisture content of the granules is between 1.5 and 3.5%, and pass through a 24-mesh sieve for granulation;
[0052] (4) Add micropowder silica gel, magnesium stearate, and the remaining amount of crospovidone to the dry granules obtained in (3), and mix well;
[0053] (5) Determining the content of intermediates, determining the weight of the tablet and pressing it to obtain the tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size range | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com