Camostat mesilate orally disintegrating tablet, and preparation method and new application thereof
A technology of camostat mesylate and orally disintegrating tablets, which is applied in the field of camostat mesylate orally disintegrating tablets and its preparation, and can solve problems such as difficulty in swallowing tablets, burning pain in the digestive tract, and high incidence of stomatitis , to achieve the effects of good promotion prospects, easy swallowing, and simple and easy preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
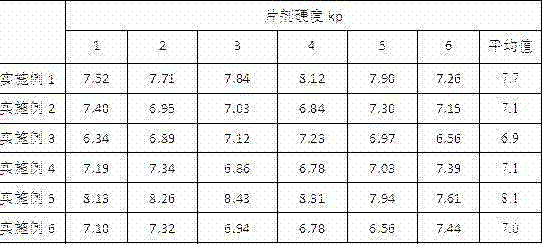
Embodiment 1
[0031] prescription:
[0032] Camostat Mesylate 20% Mannitol (Pearlitol 200 SD) 45% Sorbitol 5% Optimized Microcrystalline Cellulose (Prosolv 50) 17% Cross-linked polyvinylpyrrolidone (PVPP) 5% aspartame 2% lemon zest 2% Micropowder silica gel 3% Magnesium stearate 1%
[0033] Preparation: Grind cross-linked polyvinylpyrrolidone, aspartame, and lemon essence separately and pass through a 80-mesh sieve, and camostat mesylate pass through a 100-mesh sieve, and mix the three evenly; microcrystalline cellulose, sorbitol and mannitol Pass through a 40-mesh sieve, weigh them according to the amount, and add them to the mixed drug in step 1 in the above order and mix them evenly; then add the prescribed amount of micropowder silica gel and magnesium stearate and mix evenly, and measure the content of the intermediates to determine the content of the tablets. After re-weighting, use direct compression technology to compress...
Embodiment 2
[0035] prescription:
[0036] Camostat Mesylate 10% Mannitol (Pearlitol 50 C) 70% Sorbitol 10% Povidone (PVP-K30) 1% Cross-linked polyvinylpyrrolidone (PVPP) 6% aspartame 1% lemon zest 1% Magnesium stearate 1%
[0037] Preparation: Grind camostat mesylate, mannitol, sorbitol, cross-linked polyvinylpyrrolidone, aspartame, and lemon flavor in half of the prescription amount, respectively, and pass through a 100-mesh sieve, and mix evenly; add 2% povidone in 50% ethanol aqueous solution to prepare soft material, pass through 20 mesh sieve to granulate, dry in an oven at 40-60°C until the water content of the granules is between 2% and 4%, and the dry granules pass through 24 mesh Sieve for granulation; add magnesium stearate and the remaining amount of cross-linked polyvinylpyrrolidone to the obtained dry granules, after mixing, carry out intermediate content determination, determine the weight of the tablet, and then press ...
Embodiment 3
[0039] prescription:
[0040] Camostat Mesylate 15% Mannitol (Pearlitol 50 C) 57.2% Lactose (Granulac200) 14.3% Hydroxypropyl Cellulose (HPC-L) 2% Croscarmellose Sodium (CMC-Na) 8% aspartame 2% lemon zest 1% Magnesium stearate 0.5%
[0041] Preparation: Grind camostat mesylate, mannitol, lactose, croscarmellose sodium, aspartame, and lemon flavor in half of the prescription amount, respectively, and pass through a 100-mesh sieve, and mix evenly; the obtained mixed drug Add 2% hydroxypropyl cellulose and 25% ethanol aqueous solution to prepare soft material, pass through a 20 mesh sieve to granulate, and dry in an oven at 40-60°C until the water content of the granules is between 2% and 4%. The granules are passed through a 24-mesh sieve for granulation; magnesium stearate and the remaining amount of croscarmellose sodium are added to the obtained dry granules, after mixing, the content of intermediates is determined, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com