Method for determining trelagliptin related substances
A technology related to substances and mobile phases, which is applied in the field of chemical analysis, can solve the problems of no pharmacopoeia-listed troxagliptin succinate and no documentation, and achieve the effects of controllable related substances, scientific methods, and improved safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The present embodiment provides a method for determining related substances of trexagliptin, comprising the following steps:
[0055] S1. Preparation of the test solution;
[0056]Take trexagliptin, add solvent to dissolve and dilute to make the solution that contains succinate trexagliptin 1.0mg in every 1ml, as need testing solution; A solution containing 10 μg of trexagliptin succinate in 1 ml was used as a control solution. Inject the sample and record the chromatogram.
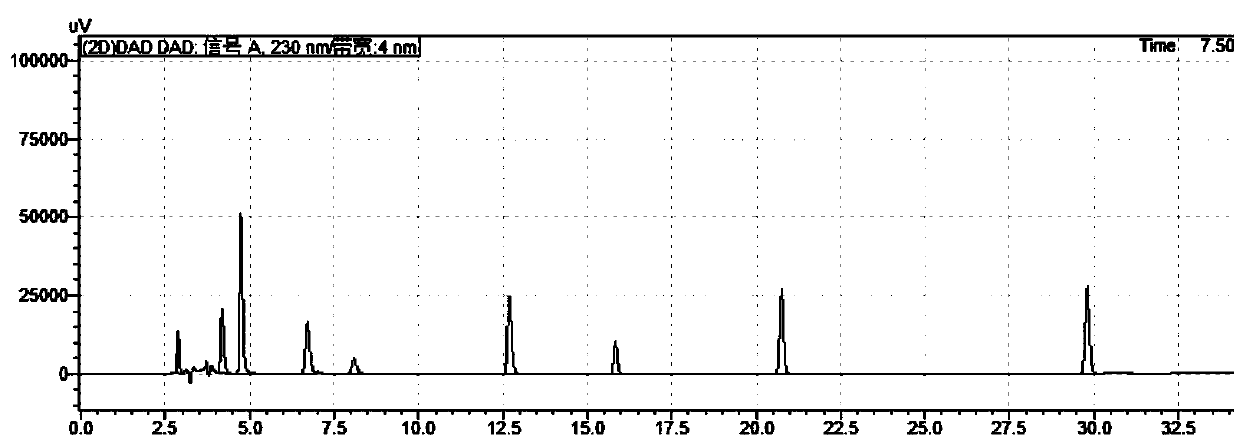
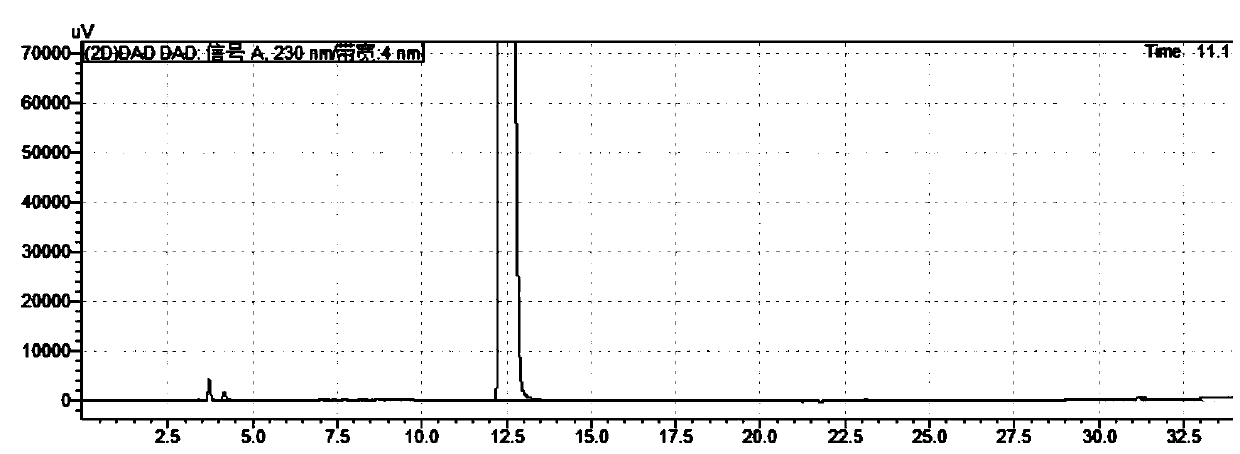
[0057] WelchUltimate AQ-C18 chromatographic column is obtained by using octadecylsilane bonded silica gel as the chromatographic column filler and filling it into the chromatographic column, and then loading and eluting. Wherein mobile phase A is phosphoric acid aqueous solution (take 1000ml of water, add phosphoric acid 1ml, pH value is 2.1), mobile phase B is acetonitrile; Flow rate: 1.0ml / min; Carry out linear gradient elution according to Table 3. For test results, see figure 1 and figure...
Embodiment 2-3
[0062] Need testing solution preparation is consistent with embodiment 1. Chromatographic conditions: mobile phase A is phosphoric acid aqueous solution (embodiment 2-0.09% phosphoric acid aqueous solution, embodiment 3-0.11% phosphoric acid aqueous solution), mobile phase B is acetonitrile; Flow rate: 1.0ml / min; Carry out linear gradient as listed in table 3 elute.
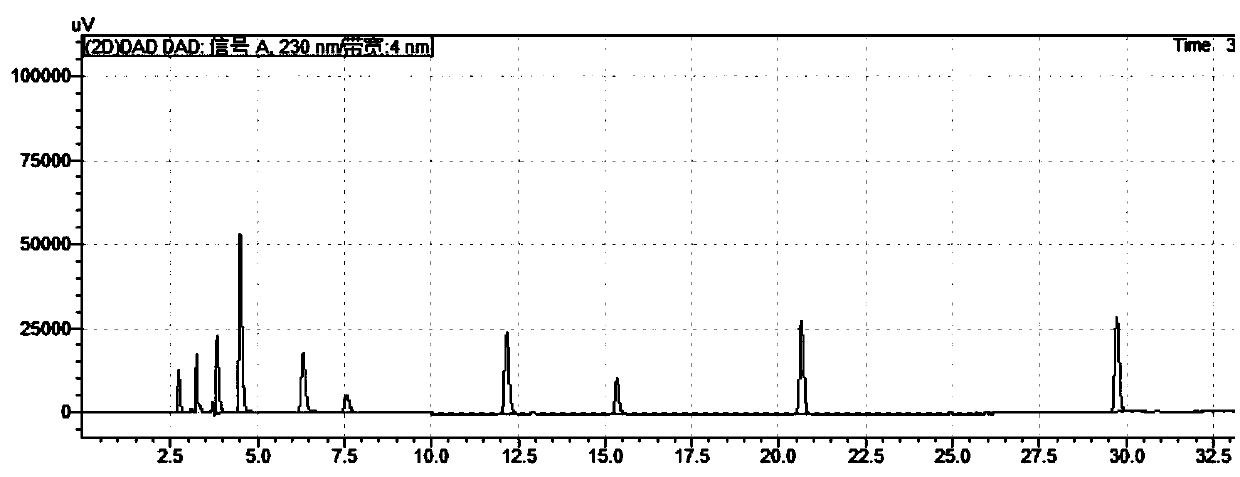
[0063] Embodiment 2 result sees attached image 3 and Figure 4 ; Embodiment 3 results are attached Figure 5 and 6 . pass image 3 , Figure 4 , Figure 5 and Figure 6 It can be seen that the condition of 0.1% phosphoric acid aqueous solution provided by the present application can meet the detection requirements, and the separation effect is good.
Embodiment 4-5
[0065] Need testing solution preparation is consistent with embodiment 1. Chromatographic conditions: mobile phase A is 0.1% phosphoric acid aqueous solution, mobile phase B is acetonitrile, the mobile phase of initial ratio (embodiment 4 initial ratio of acetonitrile is 13%, embodiment 5 initial ratio of acetonitrile is 17%); Flow velocity: 1.0ml / min; Example 4 was used for linear gradient elution in Table 4, and Example 5 was used for linear gradient elution in Table 4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com