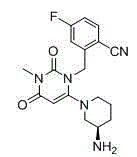
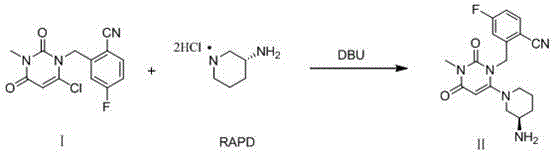
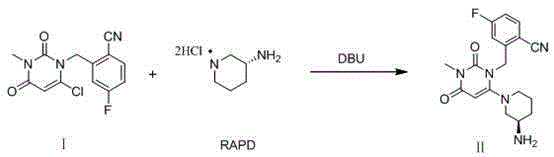
Method for preparing trelagliptin
A compound and reaction solvent technology, applied in the field of preparation of troxagliptin, can solve problems such as difficult removal, multiple impurities, and high RAPD dosage, and achieve the effects of improving yield and purity, reducing the generation of impurities and improving the reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of Trexagliptin
[0032] Add 930.0g of No. I substance, 3.0g of RAPD60, and 3.72L of acetonitrile into a 10L three-necked flask, cool down to below 10°C, and add DBU2122g dropwise. After the addition, the temperature was raised to 10-20°C, and the reaction was kept for 3 hours. Mix the reaction liquid with 15 kg of ice water, then add 5.0 L of dichloromethane, adjust the pH to 1-4 with 4M hydrochloric acid, separate the liquids, wash the water phase with 5.0 L of dichloromethane; combine the water phases, add 5.0 L of dichloromethane, Adjust the pH to 8-10 with saturated potassium carbonate solution, separate the layers, extract the aqueous phase with 5.0 L of dichloromethane, combine the organic phases, dry, filter, and concentrate to obtain the crude product No. II.
[0033] Add crude trexagliptin and 5500ml of isopropanol to the 10L reaction flask, stir and heat to reflux, and dissolve slowly. After dissolving, remove the oil bath, lower the ...
Embodiment 2
[0035] Example 2 Preparation of Trexagliptin
[0036] Add 93.0g of No. I substance, 60.3g of RAPD, and 372mL of N-methylpyrrolidone into a 1L three-necked flask, cool down to below 10°C, and add 212.2g of DBU dropwise. After the addition, the temperature was raised to 20-30°C, and the reaction was kept for 3 hours. Mix the reaction solution with 1.5kg of ice water, add 500mL of n-hexane, adjust the pH to 2-3 with 4M hydrochloric acid, separate the liquids, wash the water phase with 500mL of n-hexane; combine the water phases, add 500mL of ethyl acetate, saturated potassium carbonate solution Adjust the pH to 8-9, separate the liquids, extract the aqueous phase with 500m of ethyl acetate, combine the organic phases, dry, filter, and concentrate to obtain the crude product of No. II.
[0037] Add the crude trexagliptin and 550ml of tetrahydrofuran into the 1L reaction flask, stir and heat to reflux, and dissolve slowly. After dissolving, remove the oil bath, lower the temper...
Embodiment 3
[0039] Example 3 Preparation of Trexagliptin
[0040] Add 93.0g of No. I substance, 60.3g of RAPD, 372mL of N,N-dimethylacetamide into a 1L three-necked flask, cool down to below 10°C, and add 159.1g of DBU dropwise. After the addition, the temperature was raised to 30-40°C, and the reaction was kept for 2 hours. Mix the reaction solution with 1.5kg of ice water, add 500mL of ethyl acetate, adjust the pH to 1-2 with 4M hydrochloric acid, separate the liquids, wash the water phase with 500mL of ethyl acetate; combine the water phases, add 500mL of dichloromethane, and Adjust the pH of the sodium solution to 9-10, separate the liquids, extract the aqueous phase with 500 mL of dichloromethane, combine the organic phases, dry, filter, and concentrate to obtain the crude product No. II.
[0041] Add the crude trexagliptin and 550ml of acetonitrile into the 1L reaction flask, stir and heat to reflux, and dissolve slowly. After dissolving, remove the oil bath, lower the temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com