Preparation method of succinic acid Trelagliptin tablets
A technology of troxagliptin succinate and tablets, which is applied in the field of preparation of troxagliptin succinate tablets, can solve the problems of poor granulation effect and poor fluidity of troxagliptin succinate, and achieve good in vitro Effects of dissolution behavior, stable quality, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
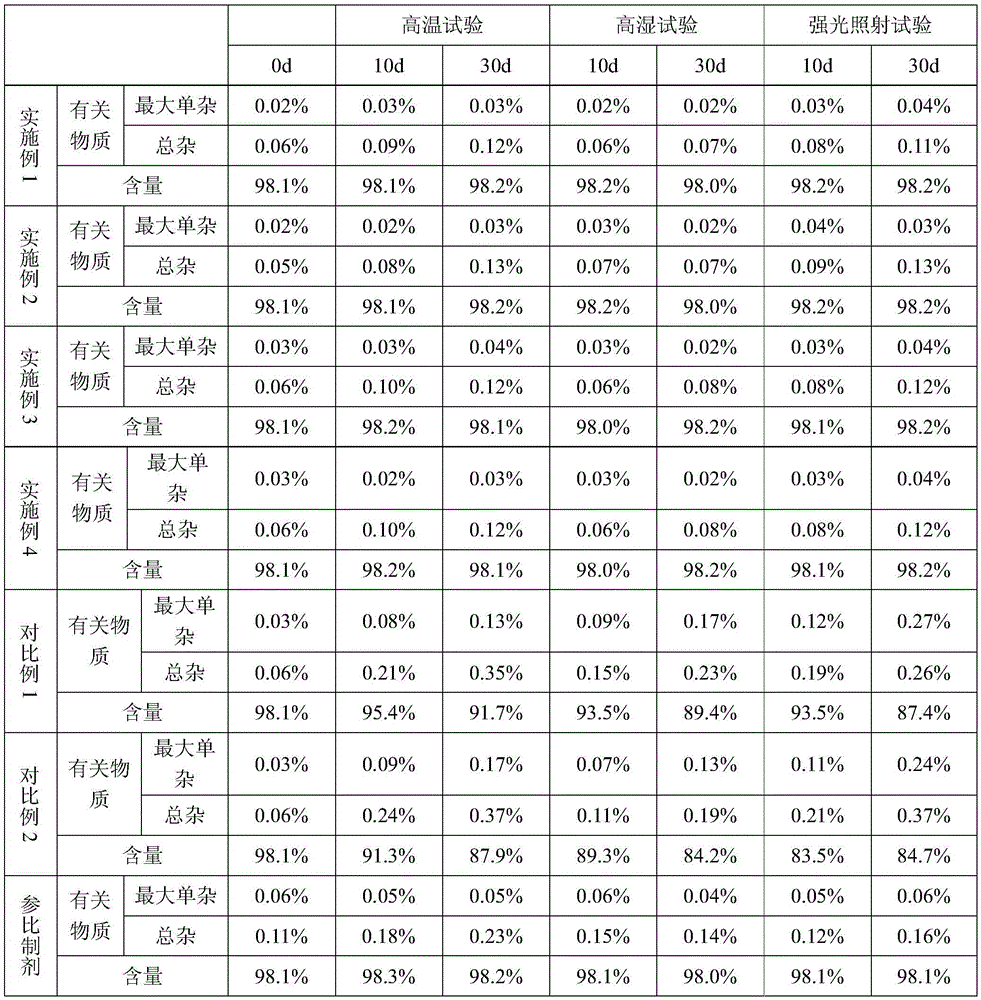
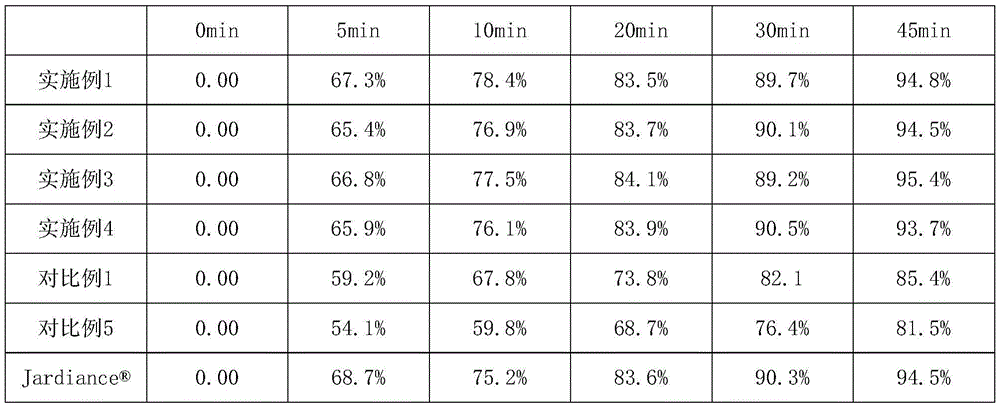
Embodiment 1
[0021] A preparation method of trexagliptin succinate tablet, is characterized in that, comprises the following steps:
[0022] Step 1, in parts by weight, take 100 parts of trexagliptin succinate, add 10 parts of sorbitol, 5 parts of microcrystalline cellulose, 6 parts of croscarmellose sodium, mix, and place the mixture at a temperature of 30 ℃ and a relative humidity of 80% for 200 minutes, then transferred to a vacuum kettle, heated to 40 ℃ under vacuum conditions, kept for 90 minutes, and discharged to obtain material 1;
[0023] Step 2, adding 1 part of adhesive in parts by weight to material 1, and wet granulation method to obtain pellets;
[0024] Step 3, drying the granules in a fluidized bed granulation coating machine to obtain dry granules;
[0025] Step 4: Add 0.8 parts by weight of lubricant to the dry granules, mix, compress into tablets, and coat to obtain trexagliptin succinate tablets.
[0026] Wherein, the particle diameters of troxagliptin succinate, sorb...
Embodiment 2
[0028] A preparation method of trexagliptin succinate tablet, is characterized in that, comprises the following steps:
[0029] Step 1, in parts by weight, take 105 parts of trexagliptin succinate, add 12 parts of sorbitol, 7 parts of microcrystalline cellulose, and 9 parts of croscarmellose sodium, mix, and place the mixture at a temperature of 35 ℃, relative humidity of 85% under the conditions of 250min, then transferred to a vacuum kettle, heated to 45℃ under vacuum conditions, kept for 70min, discharging, to obtain material 1;
[0030] Step 2, adding 3 parts of adhesive in parts by weight to material 1, and wet granulation method to obtain pellets;
[0031] Step 3, drying the granules in a fluidized bed granulation coating machine to obtain dry granules;
[0032] Step 4: Add 2 parts of lubricant in parts by weight to the dry granules, mix, compress into tablets, and coat to obtain troxagliptin succinate tablets.
[0033] Wherein, the particle diameters of trexagliptin s...
Embodiment 3
[0035] A preparation method of trexagliptin succinate tablet, is characterized in that, comprises the following steps:
[0036] Step 1, in parts by weight, take 110 parts of trexagliptin succinate, add 14 parts of sorbitol, 9 parts of microcrystalline cellulose, 10 parts of croscarmellose sodium, mix, and place the mixture at a temperature of 30 ℃ and a relative humidity of 90% for 300 minutes, then transferred to a vacuum kettle, heated to 45 ℃ under vacuum conditions, kept for 70 minutes, and discharged to obtain material 1;
[0037] Step 2, adding 3 parts of adhesive in parts by weight to material 1, and wet granulation method to obtain pellets;
[0038] Step 3, drying the granules in a fluidized bed granulation coating machine to obtain dry granules;
[0039] Step 4: Add 2.4 parts of lubricant in parts by weight to the dry granules, mix, compress into tablets, and coat to obtain trexagliptin succinate tablets.
[0040] Wherein, the particle diameters of trexagliptin succ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com